AMITROLE
| Method number: | PV2006 |
| Matrix: | Air |
| Target Concentration: | 0.2 mg/m3 (ACGIH TLV-TWA) |
| Procedure: | Samples are collected by drawing known volumes of air through impingers containing water. Samples are analyzed by high performance liquid chromatography (HPLC) using a UV detector. |
| Recommended air volume and sampling rate: | 60 L at 1 Lpm |
| Detection limit of the overall procedure (based on the recommended air volume): | 0.004 mg/m3 |
| Status of method: | Stopgap method. This method has been only partially evaluated and is presented for information and trial use. |
| Date: December 1987 (final) | Chemist: Yihlin Chan |
Carcinogen and Pesticide Branch
OSHA Analytical
Laboratory
Salt Lake City, Utah
1. General discussion
1.1. Background
The OSHA Analytical Laboratory received a set of samples requesting analysis of amitrole. The samples had been collected in impingers containing water. This report describes the preliminary validation of the sampling method and the analytical method developed for amitrole.
1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
"The oral LD50 of amitrole in mice was 11,000 or 14,700 and was 25,000 mg/kg in rats. The intravenous LD50 was 5000 mg/kg in mice. The dermal application of 10,000 mg/kg was tolerated by rats and rabbits without any systemic effects. The exposure of rats for four hours to an aerosol of 439 mg/m3 did not produce symptoms of poisoning and did not irritate the eyes and the upper respiratory tract. It has a very slight irritating effect on the skin and eyes of rabbits." (Ref. 5.5.)
The ACGIH TLV Committee recommended the removal of the A2 designation as a suspected human carcinogen from the listing for amitrole in 1984. (Ref. 5.5.)
1.3. Potential workplace exposure (Ref. 5.4.)
Amitrole is used as an herbicide and a plant growth regulator. No estimate on the extent of workplace exposure could be found.
1.4. Physical properties (Ref. 5.1. and 5.2.)
| Chemical name: | 3-Amino-1,2,4-triazole |
| Synonyms: | 3-Amino-1H-1,2,4-triazole; aminotriazole; ATA; ENT 25445; Amizol; Cytrol; Weedazol |
| CAS no. | 61-82-5 |
| Molecular formula: | C2H4N4 |
| Molecular weight: | 84.1 |
| Appearance: | Colorless crystals; bitter taste |
| Melting point: | 159°C |
| Solubility: | Soluble in water (28% at 25°C) and ethanol (26% at 75°C); slightly soluble in chloroform, methylene chloride, acetonitrile, and ethyl acetate; insoluble in ether, acetone and hydrocarbons |
| UV scan: | See Figure 3. |
| Structure: | 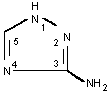 |
1.5. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 0.51 ng per injection. This is the amount of analyte which will give a peak whose height is approximately 5 times the baseline noise.
2. Sampling procedure
- 2.1. Apparatus and reagents
- 2.1.1. A personal sampling pump that can be calibrated to within
5% of the recommended flow rate
2.1.2. Impinger
2.1.3. Distilled water
2.2. Sampling procedure (Ref. 5.3.)
- 2.2.1. Calibrate pump.
2.2.2. Attach the collection device to the shirt collar or within the breathing zone. Position the tubing so as not to interfere with the work of the employee.
2.2.3. Turn on pump and record the starting time.
2.2.4. Check the pump flow periodically.
2.2.5. Prepare a blank.
2.2.6. At the end of the sampling period, turn off the pump and record the ending time.
2.2.7. Transfer the impinger solution to a vial. Rinse with a small amount water and pour the rinse solution into the vial. Seal the vial with an OSHA-21.
2.3. Recommended air volume and sampling rate
- 2.3.1. The recommended air volume is 60 L.
2.3.2. The recommended sampling rate is 1 Lpm.
2.4. Extraction efficiency
Not performed. Samples are analyzed directly.
2.5. Retention efficiency
Three impingers containing 15 mL of water were each spiked with 12.71 ug of amitrole. Humid air (80% RH, 60 L @ 1 Lpm) was drawn through the solutions. The solutions were each made 15.0 mL with water and analyzed. The average recovery was 87.5%.
| Amitrole | ||
| Sample | recovered | Recovery |
| ---------- | --------------- | ---------- |
| YCA1 | 11.30 ug | 88.9% |
| YCA2 | 10.91 ug | 85.8% |
| YCA3 | 11.17 ug | 87.9% |
| ---------- | --------------- | ---------- |
| Average = | 87.5% |
2.6. Storage
Three impingers containing 15 mL of water were each spiked with 12.71 ug of amitrole. Humid air (80% RH, 60 L @ 1 Lpm) was drawn through the solutions. The solutions were transferred to scintillation vials and stored at room temperature in the dark for 33 days. The average recovery was 79.9%.
| Amitrole | ||
| Sample | recovered | Recovery |
| ---------- | --------------- | ---------- |
| YCA4 | 9.34 ug | 73.5% |
| YCA5 | 10.48 ug | 82.5% |
| YCA6 | 10.62 ug | 83.6% |
| ---------- | --------------- | ---------- |
| Average = | 79.9% |
2.7. Interferences
There are no known interferences to the sampling procedure.
3. Analytical method
- 3.1. Apparatus
- 3.1.1. High performance liquid chromatograph
3.1.2. Chromasil Cl8 column or equivalent
3.1.3. UV detector
3.1.4. Stripchart recorder
3.2. Reagents
- 3.2.1. Water, HPLC grade
3.2.2. Amitrole, EPA #0200
3.3. Standard preparation
Weigh 5 to 10 mg of amitrole in a 10-mL volumetric flask. Add water to the mark. Dilute with water to a suitable working range.
3.4. Sample preparation
The volumes of the samples were made 15.0 mL with distilled water.
3.5. Analysis
- 3.5.1. Instrument conditions
| Column: | Chromasil C18 |
| Eluent: | 100% water |
| Flow rate: | 1.0 mL/min |
| Detector: | 205 nm |
| Injection size: | 30 uL |
| Retention time: | 6.0 min |
3.5.2. Chromatogram (See Figure 1)
3.6. Interferences
- 3.6.1. Any collected compound that has the same retention time
as amitrole and absorbs at 205 nm is a potential interference.
Generally, chromatographic conditions can be altered to separate an
interference from the analyte.
3.6.2. Retention time alone is not proof of a chemical identity. Confirmation by other means should be sought whenever possible.
3.7. Calculations
- 3.7.1. A calibration curve is constructed by plotting standard
concentrations versus detector response (see Figure 2).
3.7.2. The concentration of a sample is determined from the calibration curve.
3.7.3. The air concentration is determined by the formula:
| mg/m3 = | (µg/mL)(15 mL)
(air volume, L) |
4. Recommendations for further study
- 4.1. Investigate a more convenient sampling device, such as a
sorbent tube or a filter.
4.2. Develope a fully validated method.
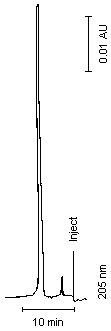
Figure 1. Chromatogram of Amitrole 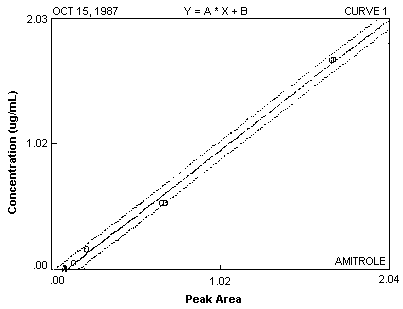
Figure 2. Calibration Curve of Amitrole 
Figure 3. UV Scan of Amitrole in Water
- 5.1. "Registry of Toxic Effects of Chemical Substances, 1983-84
Supplement"; U.S. Department of Health and Human Services: Cincinnati,
OH, 1986; DHHS(NIOSH) Publication No. 86-103.
5.2. Windholz, M., Ed. "Merck Index", 9th ed.; Merck and Co: Rahway, NJ, 1979.
5.3. "Industrial Hygiene Technical Manual", OSHA Instruction CPL 2-2.20A, March 30, 1984, U.S. Department of Labor. Chapter II: "Standard Methods for Sampling Air Contaminants."
5.4. WHO, "IARC Monograph on the Evaluation of the Carcinogenic Risk of Chemicals to Man", Volume 13; International Agency for Research on Cancer: Lyon, 1977, pp. 201-218.
5.5. "Documentation of the Threshold Limit Values and Biological Exposure Incices", Fifth Edition, American Conference of Governmental Industrial Hygienists Inc., Cincinnati, OH, 1986.