CAPROLACTAM
| Method number: | PV2012 |
| Matrix: | Air |
| Target concentration: | 1.0 mg/m3 ACGIH |
| 5.0 ppm ACGIH | |
| Procedure: | Samples are collected by drawing a known volume of air through
OSHA versatile sampler |
| Air volume and sampling rate: | 100 L and 1.0 L/min |
| Detection limit of the overall procedure (based on the recommended air volume): | 0.007 mg/m3 |
| Status of method: | Stopgap method. This method has been only partially evaluated and is presented for information and trial use. |
| Date: January 1988 (final) | Chemist: John M. Linkletter |
Carcinogen and Pesticide Branch
OSHA ANALYTICAL
LABORATORY
SALT LAKE CITY, UTAH
1. General Discussion
1.1. Background
- 1.1.1. History of procedure
This evaluation was undertaken to determine the effectiveness of
the
1.1.2. Toxic Effects (This section is for information purposes and should not be taken as the basis for OSHA policy.)
The acute oral LD50 for rats is 2140
mg/kg. The inhalation TC Lo for humans is 100 ppm. Caprolactam
(dust) has been given a
1.1.3. Potential workplace exposure
No estimate of workplace exposure was found. Caprolactam is used in the manufacture of synthetic fibers, plastics, bristles, film, coatings, synthetic leather, plasticizers, and paint vehicles; cross-linking agent for polyurethanes; synthesis of amino acid lysine. (Ref. 5.3.)
1.1.4. Physical Properties: (Refs. 5.2.-5.3.)
| Molecular Weight: | 113.18 |
| Molecular Formula: | C6H11NO |
| CAS #: | 105-60-2 |
| Melting Point: | 68-69 °C |
| Boiling Point: | 180 °C |
| Appearance: | white flakes |
| Solubility: | soluble in water, chlorinated solvents, cyclohexene. |
| Vapor Pressure: | 3 mm Hg (100 °C) 50 mm Hg (180 °C) |
| Synonyms: | Aminocaproic lactam |
| Chemical Name: | 2-oxohexamethyleneimine |
| Structure: |
1.2. Limit defining parameters
- 1.2.1. The detection limit of the analytical procedure is 3.4 ng
per injection. This is the smallest amount of analyte which will
produce a peak 5 times the baseline noise.
2. Sampling procedure
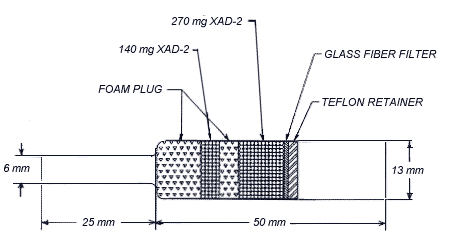
- 2.1. Apparatus
- 2.1.1. Samples are collected by using a personal sampling pump
that can be calibrated to within ± 5% of the recommended flow rate
with the sampling device in line.
2.1.2. Samples are collected with
2.2. Reagents
No sampling reagents are required.
2.3. Sampling technique
- 2.3.1. Attach the small end of the
2.3.2. After sampling for the appropriate time, remove the sampling device and seal the tube with plastic end caps.
2.3.3. Wrap each sample
2.3.4. With each set of samples, submit at least one blank. The blank should be handled the same as the other samples except that no air is drawn through it.
2.3.5. Bulks samples should be submitted for analysis in a separate container. Do not ship with the air samples.
2.4. Extraction Efficiency
Five 13 mm glass fiber filters were each liquid spiked with 127.6
ug of caprolactam. The five filters, along with a blank filter, were
each placed in separate
The average extraction efficiency for these five filters (with the XAD-7 adsorbent present) was 111%.
| Sample | Caprolactam Recovered | Recovery |
| ---------- | ---------------------------- | ------------ |
| JML-1 | 162.1 ug | 127% |
| JML-2 | 158.2 ug | 124% |
| JML-3 | 164.6 ug | 129% |
| JML-4 | 135.3 | 106% |
| JML-5 | 86.8 ug | 68% |
| ----------- | ||
| Average | 111% | |
|
| ||
2.5. Retention efficiency
Three
The average retention efficiency for these two filters was 88.3%.
| Sample | Caprolactam Spiked | Caprolactam Recovered | Recovery |
| JL-1 | 134.8 ug | 127.8 ug | 94.8% |
| JL-2 | 134.8 ug | 127.8 ug | 94.8% |
| JL-3 | 127.6 ug | 96.2 ug | 75.4% |
| ---------- | |||
| average | 88.3% | ||
2.6. Three
The average recovery after seven days of storage was 97.5%.
| Sample | Caprolactam Spiked | Caprolactam Recovered | Recovery |
| J-1 | 127.6 ug | 112.9 ug | 88.5% |
| J-2 | 134.8 ug | 134.8 ug | 100% |
| J-3 | 255.2 ug | 265.4 ug | 104% |
| --------- | |||
| average | 97.5% | ||
2.7. Recommended air volume and sampling rate
- 2.7.1. The recommended air volume is 100 L.
2.7.2. The recommended flow rate is 1.0 L/min.
2.8. Interferences
It is not known if any compounds will interfere with the collection of caprolactam. Suspected interferences should be reported to the laboratory with submitted samples.
2.9. Safety precautions
- 2.9.1. Attach sampling equipment in such a manner that it will
not interfere with work performance or safety.
2.9.2. Follow all safety practices that apply to the work area being sampled.
3. Analytical procedure
- 3.1. Apparatus
- 3.1.1. A
3.1.2. An HPLC column capable of separating caprolactam from any
interferences. A 25cm × 4.6 mm i.d.
3.1.3. An electronic integrator or other suitable means of
measuring detector response. A
3.1.4. Vials,
3.1.5. Volumetric flasks, pipets, and syringes for preparing standards, making dilutions, and performing injections.
3.2 Reagents
- 3.2.1. HPLC grade methanol.
3.2.2. HPLC grade water. A Millipore
3.2.3. Caprolactam, reagent grade.
3.3. Standard preparation
Stock standard solutions are prepared by adding methanol to pre-weighed amounts of caprolactam. Working range standard solutions are prepared by diluting stock solutions with methanol. Stock and dilute standards are stored in a freezer.
3.4. Sample preparation
- 3.4.1. Transfer each section, the
3.4.2. Add 4.0 mL of methanol to each of the three vials.
3.4.3. Seal the vials with
3.5. Analysis
- 3.5.1. Instrument conditions
| Column: | 25 cm × 4.6 mm i.d. stainless steel column, packed with 5 um LC-DB18 |
| Mobile Phase: | 25% Methanol/75% water (v/v) |
| Flow rate: | 1.1 mL/min |
| UV detector: | 218 nm |
| Retention time: | 5.5 min |
| Injection volume: | 10 uL |
3.5.2. Chromatogram (See Figure 2)
3.6. Interferences (analytical)
- 3.6.1. Any collected compound that has the same retention time
as caprolactam and absorbs at 210 and 218 nm is an interference.
Generally, chromatographic conditions can be altered to separate an
interference from the analyte.
3.6.2. Retention time on a single column is not proof of chemical identity. Analysis by an alternate HPLC column, detection at another wavelength, comparison of absorbance response ratios, and confirmation by mass spectrometry are additional means of identification.
3.7. Calculations
- 3.7.1. A calibration curve is constructed by plotting detector
response versus standard concentration.
3.7.2. The concentration of caprolactam in a sample is determined from the calibration curve. If caprolactam is found on the backup section, it is added to the amount found on the front section. Blank corrections for each section should be performed before adding the results together.
3.7.3. The air concentration is then determined by the following formula.
| mg/m3 = | (µg/mL in sample) × (extraction
volume, mL)
(air volume, L) × (extraction efficiency, decimal) |
3.8. Safety precautions
- 3.8.1. Avoid exposure to all standards.
3.8.2. Avoid exposure to all solvents.
3.8.3. Wear safety glasses at all times.
4. Recommendations for Further Study
This method should be fully validated.
5. References
- 5.1. Burright, D., Method #63, "Carbaryl (Sevin)", OSHA Analytical
Laboratory, unpublished, 1987.
5.2. "Registry of Toxic Effects of Chemical Substances", 1983-4,
Cumulative Supplement to the
5.3. "The Condensed Chemical Dictionary", Tenth ed., Van Nostrand Reinhold Co., p.191., 1983.