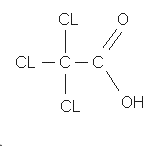
2,2-DICHLOROPROPIONIC ACID
TRICHLOROACETIC ACID
| Method number: | PV2017 |
| Matrix: | Air |
| Target concentration: | The OSHA PEL for 2,2-dichloropropionic acid is 1 ppm (6 mg/m3). The OSHA PEL for trichloroacetic acid is 1 ppm (7 mg/m3). |
| Procedure: | Samples are collected by drawing a known volume of air through a silica gel tube. Samples are desorbed with 1 mL deionized water and analyzed by high pressure liquid chromatography with an ultraviolet detector (HPLC-UV). |
| Air volume and sampling rate studied: | 10 liters at 0.2 Lpm |
| Status of method: | Stopgap method. This method has been only partially evaluated and is presented for information and trial use. |
| Date: March, 1990 | Chemist: Mary E. Eide |
SOLVENTS BRANCH
OSHA ANALYTICAL LABORATORY
SALT LAKE
CITY, UTAH
1. General Discussion
1.1. Background
1.1.1. History of procedure
The OSHA PEL for 2,2-dichloropropionic acid (DCPA) is 1 ppm (6 mg/m3). The OSHA PEL for trichloroacetic acid (TCA) is 1 ppm (7 mg/m3). Analysis by gas chromatography with an electron capture detector was attempted first, but abandoned when it was discovered that the DCPA and TCA were thermally labile. An ultraviolet scan showed a UV maximum for DCPA at 238 nm and for TCA at 236 nm. A wavelength of 229 nm was used for this study. These compounds can also be analyzed by ion chromatography. Desorption studies using charcoal tubes with various desorbing solvents were attempted, but the desorption efficiencies were less than 30%. Desorption studies using silica gel tubes were tried next and the desorption using deionized water was 100%. Retention studies showed little or no DCPA or TCA on the back-up portions. The storage at room temperature showed a decrease in recovery with time, averaging 90.0% for DCPA and 87.1% for TCA on day 12.
1.1.2. Potential workplace exposure (Ref. 5.1. and 5.2.)
Workers are exposed to DCPA and TCA in the pure form or sodium salt form in the agriculture industry where it is used as a herbicide. TCA is also used in medicine, pharmacy, and as a reagent for albumin detection.
1.1.3. Toxic Effects (This section is for information purposes and should not be taken as the basis for OSHA policy.)(Ref. 5.1. and 5.2.)
DCPA and TCA are corrosive acids, and can cause permanent eye damage. Rats exposed to a saturated atmosphere of DCPA for 7 hours showed no ill effects. Human exposure to DCPA had medical reports of injury following exposure shows mild to moderate skin, eye, respiratory, and gastrointestinal responses. Minimal respiratory irritation was found in exposures between 2 and 7 ppm DCPA. TCA showed mild to moderate skin and eye burns in medical reports of human exposure. TCA is not readily absorbed through the skin.
1.1.4. Physical properties
1.1.4.1. DCPA (Ref. 5.3.)
| Compound: | 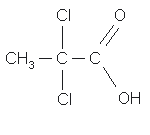 |
| Synonyms: | α-dichloropropionic acid; basfapon; dalapon; basinex; crisapon; ded-weed; devipon; dowpon; proprop; radapon; revenge; unipon |
| Molecular weight: | 142.97 |
| Density: | 1.4014 |
| Boiling point: | 99°C |
| Color: | clear liquid |
| Molecular formula: | C3H4Cl2O |
| CAS: | 75-99-0 |
| IMIS: | D176 |
| RTECS: | 67784 (UF0690000) |
| DOT: | NA 1760 |
1.1.4.2. TCA (Ref. 5.4.)
| Compound: | |||
| Synonyms: | AMCHEM; konesta; TCA; aceto-caustin; varitox; trichloroorazijnzuur | ||
| Molecular weight: | 163.38 | ||
| Melting point: | 57°C | ||
| Boiling point: | 196°C | ||
| Color: | clear liquid | ||
| Molecular formula: | C2HCl3O2) | ||
| CAS: | 76-03-9 | ||
| IMIS: | T337 | ||
| RTECS: | 2814 (AJ7875000) | ||
| DOT: | UN 1839 (solid); UN 2564 (liquid) |
1.2.1. The detection limit of the analytical procedure is 1 µg DCPA and 1 µg TCA. This is the smallest amount that could be detected under the operating conditions used in this study.
1.2.2. The overall detection limit is 0.017 ppm DCPA and 0.015 ppm TCA based on a 10 liter air volume. (All ppm amounts in this study are based on a 10 liter air volume.)
1.3. Advantages
1.3.1. The sampling procedure is convenient.
1.3.2. The analytical method is reproducible and sensitive.
1.3.3. Reanalysis of samples is possible.
1.3.4. It may be possible to analyze other compounds at the same time.
1.3.5. Interferences may be avoided by proper selection of column and LC parameters.
1.4. Disadvantages
Samples should be analyzed as soon as possible after sampling. They should be stored under refrigeration, due to the deterioration with time, as indicated in the storage study.
2. Sampling procedure
2.1. Apparatus
2.1.1. A calibrated personal sampling pump, the flow of which can be determined within + 5% at the recommended flow.
2.1.2. Silica gel tubes (20/40 mesh) containing a 150 mg adsorbing section with a 75 mg backup section separated by a 2 mm portion of urethane foam, with a silanized glass wool plug before the adsorbing section and a 3 mm plug of urethane foam at the back of the backup section. The ends are flame sealed and the glass tube containing the adsorbent is 7 cm long, with a 6 mm O.D. and 4 mm I.D., SKC tubes or equivalent.
2.2. Sampling technique
2.2.1. The ends of the silica gel tube are opened immediately before sampling.
2.2.2. Connect the silica gel tube to the sampling pump with flexible tubing.
2.2.3. Tubes should be placed in a vertical position to minimize channeling, with the smaller section towards the pump.
2.2.4. Air being sampled should not pass through any hose or tubing before entering the silica gel tube.
2.2.5. Seal the silica gel tube with plastic caps immediately after sampling. Seal each sample lengthwise with OSHA Form-21 sealing tape.
2.2.6. With each batch of samples, submit at least one blank tube from the same lot used for samples. This tube should be subjected to exactly the same handling as the samples (break ends, seal, & transport) except that no air is drawn through it.
2.2.7. Transport the samples (and corresponding paperwork) to the lab for analysis.
2.2.8. Bulks submitted for analysis must be shipped in a separate container from the air samples.
2.3. Desorption efficiency
2.3.1. Six silica gel tubes were liquid spiked at each loading of 6.92 µg (0.118 ppm), 34.6 µg (0.592 ppm), and 69.2 µg (1.18 ppm) DCPA. They were allowed to equilibrate overnight at room temperature. They were opened, each section placed into a separate 2 mL vial, desorbed with 1 mL of water, desorbed for 30 minutes with occasional shaking, and were analyzed by HPLC-UV. The overall average was 101% recovered (Table 1).
DCPA Desorption Efficiency
|
| |||||
| Tube | % Recovered | ||||
| # | 6.92 µg | 34.6 µg | 69.2 µg | ||
|
| |||||
| 1 | 102 | 100 | 105 | ||
| 2 | 97.3 | 101 | 100 | ||
| 3 | 103 | 99.0 | 100 | ||
| 4 | 105 | 102 | 101 | ||
| 5 | 100 | 104 | 102 | ||
| 6 | lost | 98.8 | 99.7 | ||
| average | 101 | 100 | 101 | ||
| overall average | 101 | ||||
| standard deviation | ± 2.18 | ||||
|
| |||||
2.3.2. Six silica gel tubes were liquid spiked at each loading of 7.09 µg (0.106 ppm), 35.4 µg (0.530 ppm), and 70.9 µg (1.06 ppm) TCA. They were allowed to equilibrate overnight at room temperature. They were opened, each section placed into a separate 2 mL vial, desorbed with 1 mL of water, desorbed for 30 minutes with occasional shaking, and were analyzed by HPLC-UV. The overall average was 100% recovered (Table 2).
TCA Desorption Efficiency
|
| |||||
| Tube | % Recovered | ||||
| # | 7.09 µg | 35.4 µg | 70.9 µg | ||
|
| |||||
| 1 | 100 | 101 | 101 | ||
| 2 | 102 | 100 | 97.4 | ||
| 3 | 97.7 | 99.1 | 99.6 | ||
| 4 | 101 | 101 | 99.8 | ||
| 5 | 101 | 102 | 100 | ||
| 6 | lost | 97.8 | 102 | ||
| average | 100 | 100 | 100 | ||
| overall average | 100 | ||||
| standard deviation | ± 1.43 | ||||
|
| |||||
2.4. Retention efficiency
2.4.1. Six silica gel tubes were spiked with 69.2 µg (1.18 ppm) DCPA allowed to equilibrate overnight, and had 10 liters humid air (93% RH) pulled through them at 0.2 Lpm. They were opened, desorbed and analyzed by HPLC-UV. There was no DCPA found on the backup portions of the tubes (Table 3). The retention efficiency averaged 101%.
DCPA Retention Efficiency
|
| |||||
| Tube # | % Recovered | % Recovered | Total | ||
| 'A' | 'B' | ||||
|
| |||||
| 1 | 100 | 0.0 | 100 | ||
| 2 | 100 | 0.0 | 100 | ||
| 3 | 103 | 0.0 | 103 | ||
| 4 | 100 | G.0 | 100 | ||
| 5 | 103 | 0.0 | 103 | ||
| 6 | 100 | 0.0 | 100 | ||
| average | 101 | ||||
|
| |||||
2.4.2. Six silica gel tubes were spiked with 70.9 µg (1.06 ppm) TCA, allowed to equilibrate overnight, and had 10 liters humid air (93% RH) pulled through them. They were opened, desorbed and analyzed by HPLC-UV. There was little or no TCA found on the backup portions of the tubes (Table 4). The retention efficiency averaged 99.5%.
TCA Retention Efficiency
|
| |||||
| Tube # | % Recovered | % Recovered | Total | ||
| 'A' | 'B' | ||||
|
| |||||
| 1 | 93.5 | 3.0 | 96.5 | ||
| 2 | 100 | 0.0 | 100 | ||
| 3 | 100 | 0.0 | 100 | ||
| 4 | 98.5 | 0.0 | 98.5 | ||
| 5 | 100 | 0.0 | 100 | ||
| 6 | 102 | 0.0 | 102 | ||
| average | 99.5 | ||||
|
| |||||
2.5. Storage
2.5.1.Silica gel tubes were spiked with 69.2 µg (1.18 ppm) DCPA and stored at room temperature until opened and analyzed. The recoveries decreased with time, averaging 93.5% for 6 days and 90.0% for 12 days stored (Table 5).
DCPA Storage Study
|
| |||
| Day | % Recovered | ||
|
| |||
| 6 | 93.3 | ||
| 6 | 93.8 | ||
| 6 | 93.5 | ||
| average | 93.5 | ||
| 12 | 89.6 | ||
| 12 | 90.4 | ||
| 12 | 89.9 | ||
| average | 90.0 | ||
|
| |||
2.5.2.Silica gel tubes were spiked with 70.9 µg (1.06 ppm) TCA and stored at room temperature until opened and analyzed. The recoveries decreased with time averaging 94.9% for 6 days and 87.3% for 12 days stored (Table 6).
TCA Storage Study
|
| |||
| Day | % Recovered | ||
|
| |||
| 6 | 97.0 | ||
| 6 | 92.5 | ||
| 6 | 95.2 | ||
| average | 94.9 | ||
| 12 | 88.1 | ||
| 12 | 86.2 | ||
| 12 | 87.6 | ||
| average | 87.3 | ||
|
| |||
2.6. Precision
2.6.1. The precision was calculated using the area counts from six injections of each standard at concentrations of 7.52, 37.6, and 75.2 µg/mL DCPA in water. The pooled coefficient of variation was 0.0127 (Table 7). The precision for TCA was measured from six injections of standards at 7.88, 39.4, and 78.8 ug/mL TCA in water. The pooled coefficient of variation was 0.00433 (Table 8).
DCPA Precision Study
|
| |||||||
| Injection | 7.52 | 37.6 | 7.52 | ||||
| Number | µg/mL | µg/mL | µg/mL | ||||
|
| |||||||
| 1 | 10486 | 60714 | 122900 | ||||
| 2 | 10420 | 60177 | 124897 | ||||
| 3 | 10429 | 61024 | 122916 | ||||
| 4 | 10759 | 60175 | 122160 | ||||
| 5 | 10336 | 60803 | 122844 | ||||
| 6 | 10808 | 61280 | 124765 | ||||
| Average | 10540 | 60696 | 123414 | ||||
| Standard | |||||||
| Deviation | ± 195 | ± 447 | ± 1134 | ||||
| CV | 0.0185 | 0.00736 | 0.00919 | ||||
| Pooled CV | 0.0127 | ||||||
TCA Precision Study
|
| |||||||||||||||
| Injection | 7.88 | 39.4 | 78.8 | ||||||||||||
| Number | µg/mL | µg/mL | µg/mL | ||||||||||||
|
| |||||||||||||||
| 1 | 17607 | 92509 | 188391 | ||||||||||||
| 2 | 17456 | 92062 | 188076 | ||||||||||||
| 3 | 17533 | 92654 | 188166 | ||||||||||||
| 4 | 17723 | 92455 | 189342 | ||||||||||||
| 5 | 17456 | 92060 | 188074 | ||||||||||||
| 6 | 17453 | 92662 | 189064 | ||||||||||||
| Average | 17538 | 92400 | 188519 | ||||||||||||
| Standard | |||||||||||||||
| Deviation | ± 109 | ± 275 | ± 549 | ||||||||||||
| CV | 0.00623 | 0.00298 | 0.00291 | ||||||||||||
| Pooled CV | 0.00433 | ||||||||||||||
where:

A(1), A(2),A(3),A(4) = # of injections at each
level
CVl, CV2, CV3, CV4 = Coefficients at each level
2.7. Air volume and sampling rate studied
2.7.1. The air volume studied is 10 liters.
2.7.2. The sampling rate studied is 0.20 liters per minute.
2.8. Interferences
Suspected interferences should be listed on sample data sheets.
2.9. Safety precautions
2.9.1. Sampling equipment should be placed on an employee in a manner that does not interfere with work performance or safety.
2.9.2. Safety glasses should be worn at all times.
2.9.3. Follow all safety practices that apply to the workplace being sampled.
3. Analytical method
3.1. Apparatus
3.1.1. High pressure liquid chromatograph equipped with an ultraviolet detector. The response is most sensitive at 236 nm. For this study a Waters M-6000A pump was used with a Waters 440 Absorbance Detector with an Extended Wavelength Module at 229 nm.
3.1.2. LC column capable of separating the analytes from any interferences. The column used in this study was 8 cm × 6.2 mm Golden series Zorbax ODS.
3.1.3. An electronic integrator or some other suitable method of measuring peak areas.
3.1.4. Four milliliter vials with Teflon-lined caps. One milliliter inserts for the four milliliter vials were used for the samples.
3.1.5. A 100 µL syringe or other convenient size for sample injection. A WISP 710 liquid autosampler was used in this study.
3.1.6. 1 mL pipets for dispensing the desorbing solution.
3.1.7. Volumetric flasks - 5, 10 mL and other convenient sizes for preparing standards.
3.1.8. Pipets- 1, 2 mL and other convenient sizes for preparing standards.
3.2 Reagents
3.2.1. Trichloroacetic acid, Reagent grade
3.2.2. 2,2-Dichloropropionic acid, 90% w/w
3.2.3. Deionized water
3.2.4. Methanol, HPLC grade
3.2.5. Phosphoric acid, Reagent grade
3.3.1. Sample tubes are opened and the front and back section of each tube are placed in separate 1 mL insets in 4 mL vials.
3.3.2. Each section is desorbed with 1 mL of deionized water.
3.3.3. The vials are sealed immediately and allowed to desorb for 30 minutes with occasional shaking.
3.4. Standard preparation
3.4.1. Stock standards are prepared by diluting a known quantity of DCPA and TCA with water.
3.4.2. At least two separate stock standards should be made.
3.4.3. Dilutions of the stock standards are made to cover the range of the samples. For this study a concentration range of 1 to 85 µg/mL DCPA and 0.7 to 90 µg/mL TCA in water was used.
3.5. Analysis
3.5.1. Liquid chromatograph conditions.
| Column: | 8 cm × 6.2 mm Golden series Zorbax ODS | ||
| Mobile Phase: | Water:methanol:phosphoric acid 75:25:0.1 at 1 mL/min | ||
| Injection size: | 40 µL | ||
| Detector: | UV at 229 nm | ||
| Chromatogram: | (See Figure 1) |
3.5.2. Peak areas are measured by an integrator or other suitable means.
3.6. Interferences (analytical)
3.6.1. Any compound having the general retention time of the analytes is an interference. Possible interferences should be listed on the sample data sheet. LC parameters should be adjusted if necessary so these interferences will pose no problems.
3.6.2. Retention time data on a single column is not considered proof of chemical identity. Samples over the target concentration should be confirmed by GC/Mass Spec or other suitable means.
3.7. Calculations
3.7.1. A curve with area counts versus concentration is calculated from the calibration standards.
3.7.2. The area counts for the samples are plotted with the calibration curve to obtain the concentration of DCPA and TCA in solution.
3.7.3. To calculate the concentration of analyte in the air sample the following formulas are used:
| (µg/m) (desorption volume)
(desorption efficiency) |
= mass of analyte in sample |
| (mass of analyte in sample)
molecular weight |
= number of moles of analyte |
| (number of moles of analyte) |
(molar volume at 25°C & 760mm) |
= | volume the analyte
will occupy at 25°C & 760mm |
| (volume analyte occupies)
(106)*
(air volume) |
= ppm |
* All units must cancel.
3.7.4. The above equations can be consolidated to form the following formula. To calculate the ppm of analyte in the sample based on a 10 liter air sample:
| (µg/mL)(DV)(24.45)(106)
(10 L)(DE)(MW) |
× | (g)
(1000 mg) |
× | (mg)
(1000 µg) |
= ppm |
| µg/mL | = | concentration of analyte in sample or standard |
| 24.45 | = | Molar volume (liters/mole) at 25° and 760 mm Hg. |
| MW | = | Molecular weight (g/mole) |
| DV | = | Desorption volume |
| 10 L | = | 10 liter air sample |
| DE | = | Desorption efficiency |
3.7.5. This calculation is done for each section of the sampling tube and the results added together.
3.8. Safety precautions
3.8.1. All handling of solvents should be done in a hood.
3.8.2. Avoid skin contact with all solvents.
3.8.3. Wear safety glasses at all times.
4. Recommendations for further study
A storage study under refrigeration should be performed. The storage study indicates a problem at ambient temperatures. Other sampling media may need to be explored to solve this problem.
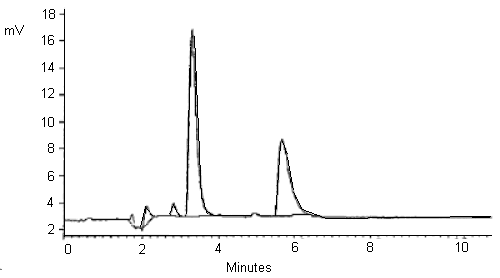
Figure 1. A standard of 75.2 µg/mL 2,2-dichloropropionic acid and
78.8 µg/mL trichloroacetic acid in water.
5. References
5.1. "Documentation of the Threshold Limit Values and Biological Exposure Indices", Fifth Edition, American Conference of Governmental Industrial Hygienists Inc., Cincinnati, OH, 1986, p. 190.
5.2. "Documentation of the Threshold Limit Values and Biological Exposure Indices", Fifth Edition, American Conference of Governmental Industrial Hygienists Inc., Cincinnati, OH, 1986, p. 592.
5.3. Windholz, M., "The Merck Index", Tenth Edition, Merck & Co., Rahway N.J., 1983, p. 450.
5.4. Windholz, M., "The Merck Index", Tenth Edition, Merck & Co., Rahway N.J., 1983, p. 1376.