DIMETHYL SUCCINATE
| Method number: | PV2021 |
| Target concentration: | 1.5 ppm (10 mg/m3) |
| Procedure: | Samples are collected by drawing a known volume of air through a charcoal tube. Samples are desorbed with 1 mL of 1:99 dimethyl formamide:carbon disulfide (DMF:CS2 ) for 30 minutes with shaking and analyzed by gas chromatography using a flame ionization detector (GC-FID). |
| Recommended air volume and sampling rate: |
20 L at 0.2 L/min |
| Reliable quantitation limit: | 0.013 ppm (0.081 mg/m3) |
| Special requirements: | Samples should be refrigerated after sampling as soon as possible, and analyzed within one week. |
| Status of method: | Partially Evaluated Method. This method has been subjected to established evaluation procedures, and is presented for information and trial use. |
| Date: October, 1995 | Chemist: Mary E. Eide |
Organic Service Branch I
OSHA Salt Lake Technical
Center
Salt Lake City, UT 84165-0200
1. General Discussion
- 1.1 Background
- 1.1.1 History
The OSHA SLTC received samples collected on charcoal tubes requesting analysis for dimethyl succinate (DMSU). A desorption study using carbon disulfide showed poor recovery, 72%, when a concentration of 448 µg DMSU was spiked on the tubes. Desorption studies using 1:99 DMF:CS2 averaged 93.8% recovery over the concentration range of 22.4 to 448 µg DMSU. The retention study showed no loss of DMSU. The storage studies had a loss of DMSU with samples collected with 20 liters humid air (80% RH at 22°C), especially those stored at room temperature, but samples stored under refrigeration had better recoveries. Storage recoveries, corrected for desorption, on day 7 were: dry refrigerated 101%, dry ambient 100%, humid refrigerated 92.8%, and humid ambient 82.2%. Storage recoveries, corrected for desorption, on day 14 were: dry refrigerated 100%, dry ambient 98.8%, humid refrigerated 86.3%, and humid ambient 76.3%. Samples should be refrigerated as soon as possible after sampling, and should be analyzed within one week of receiving them.
1.1.2 Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.) (Ref. 5.2)
DMSU is a skin, eye, and mucous membrane irritant. The Canadian recommended exposure limit for DMSU is 10 mg/m3. At the time this study was written, there was no PEL or TLV for DMSU.
1.1.3 Workplace exposure (Ref. 5.2 and 5.3)
DMSU is used as a solvent in paints, lacquers, varnishes, nitrocellulose, paint strippers, dyes, fats, photography, and waxes. DMSU is used in perfumes and flavorings for candy, ice cream, and gum. DMSU is used in the manufacture of other succinates.
1.1.4 Physical properties and other descriptive information (Ref. 5.2, 5.3, and 5.4)
| Synonyms: | Butanedioic acid, dimethyl ester; Dimethyl butanedioate; Succinic acid, dimethyl ester |
| CAS number: | 106-65-0 |
| DOT: | NA 1993 (flammable liquid) |
| IMIS: | D917 |
| RTECS: | WM7675000 |
| Molecular weight: | 146.1 |
| Flash point: | 85°C (185 °F)(cc) |
| Boiling point: | 200°C |
| Melting point: | 18°C |
| Odor: | sweet winey or fruity odor |
| Color: | clear liquid |
| Density: | 1.1198 |
| Molecular formula: | C6H10O4 |
| Structural formula: |  |
The analyte air concentrations throughout this method are based on the recommended sampling and analytical parameters. Air concentrations listed in ppm are referenced to 25°C and 101.3 kPa (760 mmHg).
- 1.2 Limit defining parameters
- 1.2.1 Detection limit of the overall procedure (DLOP)
The detection limit of the overall procedure is 0.484 µg per sample (0.00405 ppm or 0.0242 mg/m3). This is the amount of analyte spiked on the sampler that will give a response that is significantly different from the background response of a sampler blank.
The DLOP is defined as the concentration of analyte that gives a
response (YDLOP) that is significantly
different (three standard deviations
(SDBR)) from the background
response
(YBR).
The direct measurement of YBR and SDBR in chromatographic methods is typically inconvenient, and difficult because YBR is usually extremely low. inconvenient, and difficult because YBR is usually extremely low. Estimates of these parameters can be made with data obtained from the analysis of a series of samples whose responses are in the vicinity of the background response. The regression curve obtained for a plot of instrument response versus concentration of analyte will usually be linear. Assuming SDBR and the precision of data about the curve are similar, the standard error of estimate (SEE) for the regression curve can be substituted for SDBR in the above equation. The following calculations derive a formula for the DLOP:
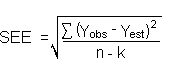
| Yobs | = | observed response |
| Yest | = | estimated response from regression curve |
| n | = | total no. of data points |
| k | = | 2 for a linear regression curve |
At point YDLOP on the regression curve
| YDLOP = A(DLOP) +YBR | A = analytical sensitivity (slope) |
therefore
| DLOP = | (YDLOP -
YBR)
A |
Substituting 3(SEE) + YBR for YDLOP gives
| DLOP = | 3(SEE)
A |
The DLOP is measured as mass per sample and expressed as equivalent air concentrations, based on the recommended sampling parameters. Ten samplers were spiked with equal descending increments of analyte, such that the lowest sampler loading was 1.12 µg/sample. This is the amount, when spiked on a sampler, that would produce a peak approximately 10 times the background response for the sample blank. These spiked samplers, and the sample blank were analyzed with the recommended analytical parameters, and the data obtained used to calculate the required parameters (A and SEE) for the calculation of the DLOP. Values of 93.7 and 15.11 were obtained for A and SEE respectively. DLOP was calculated to be 0.484 µg/sample (0.00405 ppm or 0.0242 mg/m3).
Detection Limit of the Overall Procedure
|
| |
| mass per sample | area counts |
| (µg) | (µV-s) |
|
| |
| 0 | 0 |
| 1.12 | 125 |
| 2.24 | 241 |
| 3.36 | 354 |
| 4.48 | 419 |
| 5.60 | 544 |
| 6.72 | 670 |
| 7.84 | 770 |
| 8.96 | 855 |
| 10.1 | 955 |
| 11.2 | 1085 |
|
| |

Figure 1.2.1. Plot of data to determine the DLOP/RQL.
1.2.2 Reliable quantitation limit (RQL)
The reliable quantitation limit is 1.61 µg per sample (0.013 ppm). This is the amount of analyte spiked on a sampler that will give a signal that is considered the lower limit for precise quantitative measurements.
The RQL is considered the lower limit for precise quantitative measurements. It is determined from the regression line data obtained for the calculation of the DLOP (Section 1.2.1), providing at least 75% of the analyte is recovered. The RQL is defined as the concentration of analyte that gives a response (YRQL) such that
YRQL - YBR 10(SDBR)
therefore
| RQL = | 10(SEE)
A |
RQL = 1.61µg per sample (0.013 ppm)

Figure 1.2.2. Plot of data to determine the RQL.
Reliable Quantitation Limit
|
| ||
| mass per sample | mass recovered | recovery |
| (µg) | (µg) | (%) |
|
| ||
| 1.12 | 0.981 | 87.6 |
| 2.24 | 2.09 | 93.3 |
| 3.36 | 3.20 | 95.2 |
| 4.48 | 4.31 | 96.2 |
| 5.60 | 5.23 | 93.4 |
| 6.72 | 6.40 | 95.2 |
| 7.84 | 7.59 | 96.8 |
| 8.96 | 8.43 | 94.1 |
| 10.1 | 9.47 | 93.8 |
| 11.2 | 10.5 | 93.8 |
|
| ||

Figure 1.2.3. Chromatogram of the RQL.
2. Sampling Procedure
- 2.1 Apparatus
- 2.1.1 Samples are collected using a personal sampling pump
calibrated, with the sampling device attached, to within ±5% of the
recommended flow rate.
2.1.2 Samples are collected with tubes 7 cm × 4 mm i.d. × 6 mm
o.d. glass sampling tubes packed with two sections of charcoal, lot
120. The front section contains 100 mg and the back section contains
50 mg of charcoal, lot 120. The sections are held in place with
glass wool plugs and are separated by a urethane foam plug. For this
evaluation, commercially prepared sampling tubes were purchased from
SKC Inc., (Eighty Four PA) catalog No.
2.2 Technique
- 2.2.1 Immediately before sampling, break off the ends of the
sampling tube. All tubes should be from the same lot.
2.2.2 Attach the sampling tube to the pump with flexible tubing. It is desirable to utilize sampling tube holders which have a protective cover to shield the employee from the sharp, jagged end of the sampling tube. Position the tube so that sampled air passes through the front section of the tube first.
2.2.3 Air being sampled should not pass through any hose or tubing before entering the sampling tube.
2.2.4 Attach the sampling tube vertically with the front section pointing downward, in the worker's breathing zone, and positioned so it does not impede work performance or safety.
2.2.5 After sampling for the appropriate time, remove the sample
and seal the tube with plastic end caps. Wrap each sample
2.2.6 Submit at least one blank sample with each set of samples. Handle the blank sample in the same manner as the other samples except draw no air through it.
2.2.7 Record sample volumes (in liters of air) for each sample, along with any potential interferences.
2.2.8 Ship any bulk samples separate from the air samples.
2.2.9 Submit the samples to the laboratory for analysis as soon as possible after sampling. If delay is unavoidable, store the samples in a refrigerator.
2.3 Desorption efficiency
The desorption efficiencies of DMSU were determined by
Desorption Efficiency of DMSU
|
| ||||
| % Recovery | ||||
| 0.1 X | 0.5 X | 1.0 X | 2.0 X | |
| Tube # | 22.4µg | 112µg | 224µg | 448µg |
|
| ||||
| 1 | 93.6 | 95.4 | 94.4 | 92.4 |
| 2 | 92.7 | 92.1 | 92.0 | 93.1 |
| 3 | 92.4 | 92.8 | 95.4 | 96.1 |
| 4 | 91.5 | 93.6 | 96.0 | 94.3 |
| 5 | 91.6 | 93.8 | 94.6 | 95.2 |
| 6 | 92.1 | 95.0 | 95.4 | 95.7 |
| average | 92.3 | 93.8 | 94.6 | 94.5 |
| overall average | 93.8 | |||
| standard | ±1.50 | |||
| deviation | ||||
|
| ||||
2.4 Retention efficiency
The glass wool in front of the front section of the charcoal tube
was pulled towards the end, so that none of it was in contact with the
charcoal. The glass wool was spiked with 448 µg DMSU, and the
charcoal tube had 24 L humid air (80% RH at 21°C) pulled through it at
0.2 L/min. The glass wool was spiked to determine if DMSU would
volatize off the glass wool and collect onto the charcoal. They were
opened, desorbed, and analyzed by
Retention Efficiency of DMSU
|
| ||||
| Tube # | % Recovered | |||
| Glass wool | Front section | Back section | Total | |
|
| ||||
| 1 | 0.0 | 99.3 | 0.0 | 99.3 |
| 2 | 0.0 | 99.3 | 0.0 | 99.3 |
| 3 | 0.0 | 100 | 0.0 | 100 |
| 4 | 0.0 | 100 | 0.0 | 100 |
| 5 | 0.0 | 97.8 | 0.0 | 97.8 |
| 6 | 0.0 | 95.9 | 0.0 | 95.9 |
| average | 98.7 | |||
|
| ||||
2.5 Sample storage
The front sections of twelve sampling tubes were each spiked with 448 µg (3.75 ppm) of DMSU, then six tubes were stored in the refrigerator (-10°C), and six were stored at room temperature 23°C. Twelve more tubes were spiked with 448 µg DMSU, and had 20 liters of humid air (80% RH at 21°C) drawn through them, before six tubes were stored in the refrigerator (-10°C), and six were stored at room temperature 23°C. Three of each type of samples were analyzed after 7 days and the remaining three samples of each type after 14 days. The amounts recovered indicate that humidity and temperature affect the ability of charcoal to retain intact the DMSU. The recoveries decreased with time and/or added humidity, with the worst recovery on day 14 day storage with humidity. Results are corrected for desorption efficiency.
Storage Test for DMSU
|
| ||||
| Time (days) |
%Recovery Humid Ambient |
%Recovery Humid Refrigerated |
%Recovery Dry Ambient |
%Recovery Dry Refrigerated |
|
| ||||
| 7 | 79.6 | 93.5 | 101 | 101 |
| 7 | 79.7 | 92.3 | 101 | 101 |
| 7 | 87.3 | 92.6 | 98.8 | 100 |
| average | 82.2 | 92.8 | 100 | 101 |
| 14 | 74.5 | 87.0 | 96.7 | 101 |
| 14 | 76.1 | 86.1 | 98.6 | 100 |
| 14 | 78.5 | 85.7 | 101 | 100 |
| average | 76.3 | 86.3 | 98.8 | 100 |
|
| ||||
2.6 Recommended air volume and sampling rate.
Based on the data collected in this evaluation, 20 L air samples should be collected at a sampling rate of 0.2 L/min.
2.7 Interferences (sampling)
- 2.7.1 It is not known if any compounds will severely interfere
with the collection of DMSU on the sampling tubes. In general, the
presence of other contaminant vapors in the air will reduce the
capacity of the charcoal tube to collect DMSU.
2.7.2 Suspected interferences should be reported to the laboratory with submitted samples.
2.8 Safety precautions (sampling)
- 2.8.1 Attach the sampling equipment to the worker in such a
manner that it will not interfere with work performance or safety.
2.8.2 Follow all safety practices that apply to the work area being sampled.
2.8.3 Wear eye protection when breaking the ends of the glass sampling tubes.
3. Analytical Procedure
- 3.1 Apparatus
- 3.1.1 The instrument used in this study was a gas chromatograph
equipped with a flame ionization detector, specifically a Hewlett
Packard model 5890.
3.1.2 A GC column capable of separating the analyte from any
interferences. The column used in this study was a 60 meter
capillary column with a 0.5 µm coating of
3.1.3 An electronic integrator or some suitable method of measuring peak areas.
3.1.4 Two milliliter vials with TeflonTM-lined caps.
3.1.5 A 10µL syringe or other convenient size for sample injection.
3.1.6 Pipets for dispensing the desorbing solution. A Repipet® dispenser was used in this study.
3.1.7 Volumetric flasks - 5 or 10 mL and other convenient sizes for preparing standards.
3.2 Reagents
- 3.2.1 GC grade nitrogen, hydrogen, and air.
3.2.2 Dimethyl succinate (DMSU), Reagent grade
3.2.3 Carbon disulfide (CS2), Reagent grade
3.2.4 Dimethyl formamide (DMF), Reagent grade
3.2.5 p-Cymene (internal standard), Reagent grade
3.2.6 Desorbing solution was 1:99 DMF:carbon disulfide with 0.25 µL/mL p-cymene internal standard.
3.3 Standard preparation
- 3.3.1 At least two separate stock standards are prepared by
diluting a known quantity of DMSU with the desorbing solution of
1:99 DMF:carbon disulfide with 0.25 µL/mL p-cymene
internal standard. The concentration of these stock standards was
0.2 µL/mL or 224 µg/mL.
3.3.2 A third standard at a higher concentration was prepared to check the linearity of the calibration. For this study, two analytical standards were prepared at a concentration of 0.2 µL/mL (224 µg/mL), and one at 1.0 µL/mL (1120 µg/mL) DMSU in the desorbing solution.
3.4 Sample preparation
- 3.4.1 Sample tubes are opened and the front and back section of
each tube are placed in separate 2 mL vials.
3.4.2 Each section is desorbed with 1 mL of the desorbing solution of 1:99 DMF:carbon disulfide with 0.25 µL/mL p-cymene internal standard.
3.4.3 The vials are sealed immediately and allowed to desorb for 30 minutes with constant shaking.
3.5 Analysis
- 3.5.1 Gas chromatograph conditions.
| Injection size: | 1 µL | |
| Flow rates (mL/min) | ||
| Nitrogen (make-up): | 30 | |
| Hydrogen(carrier): | 2 | |
| Hydrogen(detector): | 40 | |
| Air: | 420 | |
| Temperatures (°C) | ||
| Injector: | 200 | |
| Detector: | 220 | |
| Column: | 50° for 2 min then 10°/min to 170° for 15 min | |

Figure 3.5.1 Chromatogram of an analytical standard at the target
concentration. Peak identification: (1) carbon disulfide,
3.5.2 Peak areas are measured by an integrator or other suitable means.
3.6 Interferences (analytical)
- 3.6.1 Any compound that produces a response and has a similar
retention time as the analyte is a potential interference. If any
potential interferences were reported, they should be considered
before samples are desorbed. Generally, chromatographic conditions
can be altered to separate an interference from the analyte.
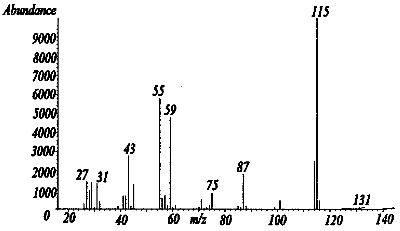
Figure 3.6.1 A mass spectra of dimethyl succinate
(DMSU).
3.6.2 When necessary, the identity or purity of an analyte peak
may be confirmed by
3.7 Calculations
- 3.7.1 The instrument was calibrated with a standard of 224
µg/mL DMSU in the desorbing solution. The linearity of the
calibration was checked with a standard of 1120 µg/mL.
3.7.2 If the calibration is
3.7.3 To calculate the concentration of analyte in the air sample the following formulas are used:
| (µg/m) (desorption volume)
(desorption efficiency) |
= mass of analyte in sample |
| (mass of analyte in sample)
molecular weight |
= number of moles of analyte |
| (number of moles of analyte) |
(molar volume at 25°C & 760mm) |
= | volume the analyte
will occupy at 25°C & 760mm |
| (volume analyte occupies)
(106)*
(air volume) |
= ppm |
* All units must cancel.
3.7.4 The above equations can be consolidated to the following formula.
| (µg/mL)(DV)(24.46)(106)(g)(mg)
(20 L)(DE)(MW)(1000mg)(1000µg) |
= ppm |
| µg/mL | = | concentration of analyte in sample or standard |
| 24.46 | = | Molar volume (liters/mole) at 25° and 760 mm Hg. |
| MW | = | Molecular weight (g/mole) |
| DV | = | Desorption volume |
| 20 L | = | 20 liter air sample |
| DE | = | Desorption efficiency |
3.7.5 This calculation is done for each section of the sampling tube and the results added together.
3.8 Safety precautions (analytical)
- 3.8.1 Avoid skin contact and inhalation of all chemicals.
3.8.2 Wear safety glasses, gloves and a lab coat at all times while in the laboratory areas.
4. Recommendations for Further Study
Collection studies need to be performed from a dynamically generated test atmosphere. Other sampling medias should be explored to find one that will provide better storage stability.
5. References
- 5.1 Trade names Database on CCINFO
5.2 Lide, D.R., "Handbook of Chemistry and Physics", 73rd Edition, CRC Press Inc., Boca Raton FL, 1992, p. 3-470.
5.3 Windholz, M., "The Merck Index", Eleventh Edition, Merck & Co., Rahway N.J., 1989, p. 1399.