
GASOLINE
| Method number: | PV2028 |
| Matrix: | Air |
| Target concentration: | 900 mg/m3 (300 ppm) (ACGIH TWA-TLV) |
| Procedure: | Samples are collected by drawing a known volume of air through a charcoal tube. Samples are desorbed with carbon disulfide and analyzed by gas chromatography using a flame ionization detector. |
| Air volume and sampling rate studied: |
10 L at 0.1 L/min |
| Status of method: | Stopgap method. This method has been only partially evaluated and is presented for information and trial use. |
| Date: December 1, 1987 | Chemist: Wayne Potter |
SOLVENTS BRANCH
OSHA ANALYTICAL LABORATORY
SALT LAKE
CITY, UTAH
1. General Discussion
- 1.1. Background
- 1.1.1. History of procedure
The OSHA Laboratory recently received a set of samples collected on SKC Lot 120 charcoal tubes requiring analysis for gasoline. Similar compounds have been collected on charcoal tubes, desorbed with carbon disulfide and analyzed by gas chromatography using a flame ionization detector.
1.1.2. Toxic Effects (This section is for information purposes and should not be taken as the basis for OSHA policy.)
Ingestion of gasoline causes inebriation, vomiting, vertigo, fever, drowsiness, confusion and cyanosis. Aspiration of gasoline causes bronchitis or pneumonia. Inhalation of gasoline causes intense burning in throat and lungs; possibly bronchopneumonia. Exposure to the skin can cause slight burning, itching sensations and increased desquamation. (Ref. 5.1.)
1.1.3. Potential workplace exposure
Occupational exposure can occur in the manufacture, transportation and distribution of gasoline. This includes truck drivers, marine loading operators and service station attendants. No estimate was found as to the number of workers exposed annually.
1.1.4. Physical properties: (Ref. 5.1.)
| Compound: | Gasoline |
| CAS: | 8006-61-9 |
| RTECS: | LX3300000 |
| IMIS: | 1340 |
| Specific gravity: | 0.72 to 0.76 at 60/60°F |
| Boiling point: | Initial BP 39°C; after 10% distilled BP 60°C; after 50% distilled BP 110°C; after 90% distilled BP 170°C; final BP 204°C. |
| Solubility: | Insoluble in water; freely soluble in alsolute alcohol, ether, chloroform and benzene. |
| Flash point: | about (-45°C) |
| Description: | Colorless mobile liquid with characteristic odor. Mixture of C4 to C12 hydrocarbons. The major components are branched-chain paraffins, cycloparaffins and aromatics. Highly flammable; dangerous fire and explosion risk. |
1.2. Limit defining parameters
- 1.2.1. The detection limit of the analytical procedure is 9.0 µg
per injection. This is the amount of gasoline which will produce a
few characteristic peaks at least 5 times the baseline noise.
1.3. Advantages
- 1.3.1. The sampling procedure is convenient.
1.3.2. The analytical method is reproducible and sensitive.
1.3.3. Reanalysis of samples is possible.
1.3.4. It may be possible to analyze other compounds at the same time.
1.3.5. Interferences may be avoided by proper selection of column and GC parameters.
1.4. Disadvantages
- 1.4.1. Confirmation of low levels of gasoline may be difficult
due to similarities with low boiling fractions of other petroleum
products.
2. Sampling procedure
- 2.1. Apparatus
- 2.1.1. A calibrated personal sampling pump, the flow of which
can be determined within ±5% at the recommended flow.
2.1.2. Coconut shell charcoal tubes which consist of glass tubes 7 cm long, 6-mm OD, and 4-mm ID, containing a 100-mg section and a 50-mg section of charcoal separated with a urethane foam plug are used. The glass tube is flame sealed at both ends. For this evaluation, SKC, Inc. charcoal tubes, lot 120, were used.
2.2. Sampling technique
- 2.2.1. The ends of the charcoal tubes are opened immediately
before sampling.
2.2.2. Connect the charcoal tubes to the sampling pump with flexible tubing.
2.2.3. Tubes should be placed in a vertical position to minimize channeling, with the smaller section towards the pump.
2.2.4. Air being sampled should not pass through any hose or tubing before entering the charcoal tube.
2.2.5. Seal the charcoal tubes with plastic caps immediately after sampling. Seal each sample lengthwise with an OSHA Form-21.
2.2.6. With each batch of samples, submit at least one blank tube from the same lot used for samples. This tube should be subjected to exactly the same handling as the samples (break ends, seal, & transport) except that no air is drawn through it.
2.2.7. Ship the samples (and corresponding paperwork) to the lab for analysis.
2.2.8. Bulks submitted for analysis must be shipped in a separate container from the samples.
2.3. Desorption efficiency
Six charcoal tubes were spiked with gasoline at 1/10, 1/2, and 2 times the target concentration and refrigerated overnight. The charcoal was desorbed with 1 mL carbon disulfide and analyzed by gas chromatography with a flame ionization detector. The results shown in Table 2 averaged 99.0% recovery over the range studied.
Desorption Efficiency
% Desorption
|
| |||
| Sample Number |
2 × 17.51 mg |
0.5 × 4.376 mg |
0.1 × .875 mg |
|
| |||
| 1 2 3 4 5 6 |
104.7% 100.0 104.6 105.9 104.6 106.1 |
100.0% 100.0 99.5 101.2 98.8 98.3 |
91.5% 87.8 96.4 91.1 98.6 92.3 |
| Average | 104.3 | 99.6 | 93.0 |
| Standard Deviation |
± 2.22 | ± 1.02 | ± 3.91 |
| Overall Average |
98.9 | ||
|
| |||
2.4. Retention efficiency
Six charcoal tubes were spiked at 2 times the target concentration with gasoline. Ten liters of humid air (about 50% relative humidity) were drawn through each tube at 0.1 Lpm. The tubes were stored in a refrigerator overnight. The tubes were desorbed with 1 mL of carbon disulfide and analyzed by gas chromatography with a flame ionization detector. The retention averaged 100.9%. (Table 2)
Retention Efficiency
|
| |||
| Number | Sample Volume |
Air Spiked (mg) |
Amount Retained |
|
| |||
| 1 2 3 4 5 6 |
10 L 10 L 10 L 10 L 10 L 10 L |
17.51 17.51 17.51 17.51 17.51 17.51 |
101.0 103.6 100.6 101.6 99.0 99.6 |
| Average | 100.9 | ||
|
| |||
2.5. Storage
Storage samples were generated by spiking six charcoal tubes with gasoline, 8.753 mg, and then pulling 10 L of humid air through them at about 50% relative humidity. The samples were stored for three days in a refrigerator before analysis. There was an average recovery of 98.1% from the tubes (Table 3).
Storage Stability
|
| ||||
| Sample Number |
Air Volume |
Amount Spiked (mg) |
Days Stored |
% Recovered |
|
| ||||
| 1 2 3 4 5 6 |
10.0 L 10.0 L 10.0 L 10.0 L 10.0 L 10.0 L |
8.753 8.753 8.753 8.753 8.753 8.753 |
3 3 3 3 3 3 |
96.8 97.7 97.7 99.4 98.0 99.2 |
| Average | 98.1 | |||
|
| ||||
2.6. Air volume and sampling rate studied were 10 L and 0.1 L/min.
2.7. Interferences
Suspected interferences should be listed on sample data sheets.
2.8. Safety precautions
- 2.8.1. Sampling equipment should be placed on an employee in a
manner that does not interfere with work performance or safety.
2.8.2. Safety glasses should be worn at all times.
2.8.3. Follow all safety practices that apply to the workplace being sampled.
3. Analytical method
- 3.1. Apparatus
- 3.1.1. Gas chromatograph equipped with a flame ionization
detector.
3.1.2. GC column capable of separating the analyte and an internal standard from any interferences. A 60 meter DB-wax fused silica capillary column was used in this evaluation.
3.1.3. An electronic integrator or some other suitable method of measuring peak areas.
3.1.4. Two milliliter vials with TeflonTM-lined caps.
3.1.5. A 5 µL syringe or other convenient size for sample injection.
3.1.6. A device for dispensing the desorbing solution. The Glenco 1 mL dispenser was used in this evaluation.
3.1.7. Volumetric flasks - 5 mL and other convenient sizes for preparing standards.
3.1.8. A syringe for standard preparation (10-50 µL).
3.2. Reagents
- 3.2.1. Purified GC grade nitrogen, hydrogen, oxygen, and air.
3.2.2. Carbon Disulfide, reagent grade.
3.2.3. Internal Standard; p-Cymene was used in this evaluation.
3.3. Sample preparation
- 3.3.1. Sample tubes are opened. The front and back sections of
charcoal in each tube are placed in separate 2 mL vials.
3.3.2. Each section is desorbed with 1 mL of carbon disulfide.
3.3.3. The vials are sealed immediately and allowed to desorb for 30 minutes with occasional shaking.
3.4. Standard preparation
- 3.4.1. Standards are prepared by diluting a known quantity of
gasoline with the desorbing solution of carbon disulfide. A sample
chromatogram is presented in Figure
1.
3.4.2. At least two separate standards should be made.
3.5. Analysis
- 3.5.1. Gas chromatograph conditions.
| Flow rates (mL/min) | Temperature (°C) | ||
| Nitrogen: | carrier 3 makeup 27 |
Injector: | 175 |
| Hydrogen: | 30 | Detector: | 200 |
| Air: | 240 | Column: | 60-160 |
| Injection size: | 1 µL | Rate: | 10°/min |
| Chromatogram: | (See Figure 1) | ||
| Attenuation: | 4 | ||
| Range: | 2 | ||
3.5.2. Peak areas are measured by an integrator or other suitable means.
- 3.5.2.1. The data system used in this evaluation was a
3.5.2.2. The areas of the peaks due to gasoline constituents are added together (area summation) in the analysis of the standards and samples. The summed areas and the concentration of the analytical standards are used to determine a linear least squares fit equation. The concentration of the samples is determined by entering their summed areas into the least squares equation.
3.5.2.3. If the peaks present in the samples do not elute in approximately the same time range as the standards, a comparison of the constituents in the samples and standard should be done by mass spec to confirm that the samples do contain gasoline type compounds. If distinct analytes are confirmed by mass spec, their identity and approximate concentration should be reported.
3.5.4. Precision
The precision of the analytical method was evaluated by doing multiple injections of gasoline standards. The pooled coefficient of varaition over the range of 0.1 to 2 times the target concentration was 0.019. (Table 5)
Precision
|
| ||||
| Injection Number |
2. × 17.506 mg/mL |
1. × 8.753 mg/mL |
0.5 × 4.376 mg/mL |
0.1 × .875 mg/mL |
|
| ||||
| 1 2 3 4 5 6 |
99.8 99.0 100.2 99.5 100.1 98.5 |
103.6 104.4 102.4 101.2 101.5 101.1 |
100.3 102.1 103.5 103.2 102.4 105.9 |
100.4 96.1 99.6 100.7 94.6 94.4 |
| Average | 99.5 | 102.4 | 102.9 | 97.6 |
| Standard Deviation |
.662 | 1.37 | 1.85 | 2.93 |
| CV | .0067 | .013 | .018 | .030 |
| Pooled CV | 0.019 | |||
|
| ||||
| Coefficient of variation (CV) = | standard
deviation average |

A1, A2, A3, A4 = # injections at each level
CV1, CV2, CV3, CV4
= Coefficients at each level
3.6. Interferences (analytical)
- 3.6.1. Possible interferences should be listed on the sample
data sheet. GC parameters should be adjusted if necessary so these
interferences will pose no problems, although a mixture such as
gasoline poses difficulties.
3.6.2. Retention time data on a single column is not considered proof of chemical identity. Samples over the target concentration should be confirmed by GC/Mass Spec or other suitable means.
3.7. Calculations
- 3.7.1. Gasoline should be reported as mg/m3 since any
ppm value would require the use of an approximate molecular weight.
3.7.2. The air concentration in mg/m3 is determined from the mass of gasoline in the sample. The following equation is used to calculate the mg/m3 of gasoline based on a 10 liter air sample, and 1 mL desorbing solution:
| (ug) (1 mL) (L) (DE) |
= mg/m3 |
| ug | = | Mass of analyte in the sample |
| 1 mL | = | Desorption volume |
| L | = | Air volume |
| DE | = | Desorption efficiency |
3.8. Safety precautions
- 3.8.1. All handling of solvents should be done in a hood.
3.8.2. Avoid skin contact with all solvents.
3.8.3. Wear safety glasses at all times.
4. Recommendations for further study
- 4.1. Longer storage tests should be performed.
4.2. Further work should be done to fully validate the method.
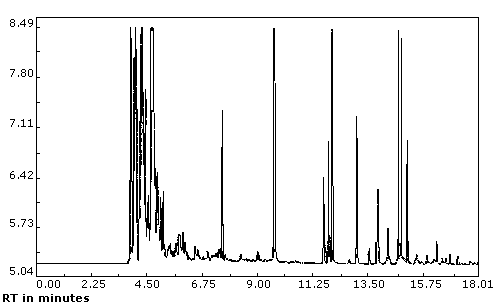
Figure 1
5. References
- 5.1. Windholz M., Ed.; Merck Index, 10th
ed.; Merck and Co: Rahway, NJ, 1983, p 624.