HDI BIURET (BIURET OF HEXAMETHYLENEDIISOCYANATE)
| Method number: | PV2030 | |
| Matrix: | Air | |
| Target Concentration: | 0.43 mg/m3 or 0.02 ppm (arbitrary). There is no OSHA PEL or ACGIH TLV for HDI biuret. | |
| Procedure: | Samples are collected by drawing a known volume of air
through glass fiber filters coated with 1 mg of
| |
| Recommended air volume and sampling rate: | 15 L at a flow of 1 L/min | |
| Detection limit of the overall procedure (based on the recommended air volume): | 0.02 mg/m3 | |
| Status of method: | Stopgap method. This method has been only partially evaluated and is presented for information and trial use. | |
| Date: January 1988(final) | Chemists: | David B. Armitage |
| Yihlin Chan | ||
| George F. Lewis | ||
Carcinogen and Pesticide Branch
OSHA Analytical
Laboratory
Salt Lake City, Utah
1. General discussion
1.1. Background
The growing concern with workers' exposure to a variety of
isocyanates has spurred a demand for the analysis of work site
atmosphere for HDI biuret. OSHA Analytical Laboratory had so far
validated the sampling and analytical methods for MDI,
HDI biuret is manufactured by treating HDI with water under
controlled conditions. Pure HDI biuret is not commercially available.
Mobay's Desmodur 100 is a mixture of
1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
No toxicity data of HDI biuret was found in TOXNET or TOXLINE data bases. NIOSH reported increase in symptoms of eye irritation, nasal irritation, throat irritation and chest discomfort among workers engaged in spray painting. These workers were found to be exposed to significant concentrations of organic solvents and isocyanates compound (HDI and HDI biuret). (Ref. 5.1.)
1.3. Potential workplace exposure
No data on the extent of worker exposure to AD1 biuret could be found. The type of work most liable to HDI biuret exposure is spray painting with polyurethane paints -- for example, of airplanes (Ref. 5.1.) and of cars (Ref. 5.2.).
1.4. Physical properties (Ref. 5.4.)
| Chemical name: | N,N',2-Tris(6-isocyanatohexyl)imidodicarbonic diamide |
| Synonyms: | HDI biuret |
| HDI-biuret | |
| biuret of | |
| Desmodur 100 | |
| CAS no. | 4035-89-6 |
| Molecular formula: | C23H38N6O5 |
| Molecular weight: | 478 |
| Melting point: | -19 °C |
| Density: | 1.114(40/4) |
| Structure: | 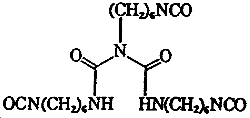 |
1.5. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 0.47 ng per injection. This is the amount of analyte which will give a peak whose height is approximately 5 times the baseline noise.
2. Sampling procedure
2.1. Apparatus and reagents
2.1.1. A personal sampling pump that can be calibrated to within 5% of the recommended flow rate
2.1.2.
2.1.3. Filter holder (cassette) for
2.2. Sampling procedure (Ref. 5.5.)
2.2.1. Calibrate pump. Remove the inlet cover from the
2.2.2. Attach the collection device to the shirt within the breathing zone. Position the excess tubing so as not to interfere with the work of the employee.
2.2.3. Turn on pump and record the starting time.
2.2.4. Check the pump flow periodically.
2.2.5. Prepare a blank. The blank should be treated the same way as the samples except no air was drawn through it.
2.2.6. At the end of the sampling period, turn off the pump and record the ending time.
2.2.7. Replace the cover and seal the casette with an
2.3. Recommended air volume and sampling rate
2.3.1. The recommended air volume is 15 L.
2.3.2. The recommended sampling rate is 1 L/min.
2.4. Extraction efficiency
Three
| Derivatized | ||
| HDI biuret | ||
| Sample | recovered | Recovery |
| ---------- | --------------- | ---------- |
| YC13 | 1.863 ug | 97.0% |
| YC14 | 1.934 ug | 100.7% |
| YC15 | 2.080 ug | 108.3% |
| ---------- | --------------- | ---------- |
| Average = | 102.0% |
2.5. Retention efficiency
Three
| Derivatized | ||
| HDI biuret | ||
| Sample | recovered | Recovery |
| ---------- | --------------- | ---------- |
| YC16 | 1.894 ug | 98.6% |
| YC17 | 2.104 ug | 109.6% |
| YC18 | 2.019 ug | 105.2% |
| ---------- | --------------- | ---------- |
| Average = | 104.5% |
2.6. Storage
Three
| Derivatized | ||
| HDI biuret | ||
| Sample | recovered | Recovery |
| ---------- | --------------- | ---------- |
| YC19 | 1.908 ug | 99.4% |
| YC20 | 1.843 ug | 96.0% |
| YC21 | 1.817 ug | 94.6% |
| ---------- | --------------- | ---------- |
| Average = | 96.7% |
2.7. Interferences
Compounds such as anhydrides, acid chlorides, and other isocyanates
that react with
3. Analytical method
3.1. Apparatus
3.1.1. High performance liquid chromatograph
3.1.2. Nucleosil Cl8 column or equivalent
3.1.3. UV or fluorescence detector
3.1.4. Stripchart recorder
3.2. Reagents
3.2.1. Water, HPLC grade
3.2.2. Acetonitrile, HPLC grade
3.2.3. Dimethyl sulfoxide, reagent grade
3.2.4. HDI biuret, purified (see below)
3.2.5.
3.2.6.
3.2.7. Phosphoric acid, reagent grade
3.3. Standard preparation
3.3.1. Preparation and purification of the
Desmodur 100 1.17 g was dissolved in 30 mL of DMSO.
3.3.2. Preparation of standard solution
Weigh 3 to 5 mg of the purified HDI biuret
3.4. Sample preparation
Samples were extracted with 4.0 mL of 90/10 (v/v) ACN/DMSO by shaking for 30 minutes on a mechanical shaker.
3.5. Analysis
3.5.1. Instrument conditions
| Column: | Nucleosil C18 10 um | |
| Eluent: | 57% acetonitrile, 43% water, 0.01 M di-n-butylamine, phosphoric acid to pH 5.3 | |
| Flow rate: | 1.6 mL/min | |
| Detector: | Fluorescence: | excitation 240 nm |
| emission 370 nm | ||
| UV: | 254 nm | |
| Injection size: | 10 uL | |
| Retention time: | 9.2 min | |
3.5.2. Chromatograms (See Figure 2)
3.6. Interferences
3.6.1. Any collected compound that has the same retention time as HDI biuret and responds to the detector is a potential interference. Generally, chromatographic conditions can be varied to separate an interference from the analyte.
3.6.2. Retention time alone is not proof of a chemical identity. Confirmation by other means should be sought whenever possible.
3.7. Calculations
3.7.1. A calibration curve for HDI biuret is constructed by plotting standard concentrations versus detector response (see Figure 4).
3.7.2. The concentration of HDI biuret for a sample is determined from the calibration curve.
3.7.3. The air concentration is determined by the formula:
| mg/m3 = | (µg/mL)(4 mL)
(air volume, L)(extraction efficiency) |
4. Recommendations for further study
4.1. The method should be fully validated.
4.2. Validation should also be conducted at the levels of the field samples.
4.3. Alternative methods of establishing the purity of HDI biuret should be investigated.
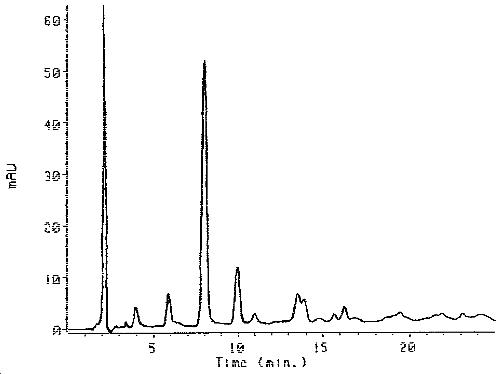
Figure 1. Chromatogram of the Derivatized Desmodur 100
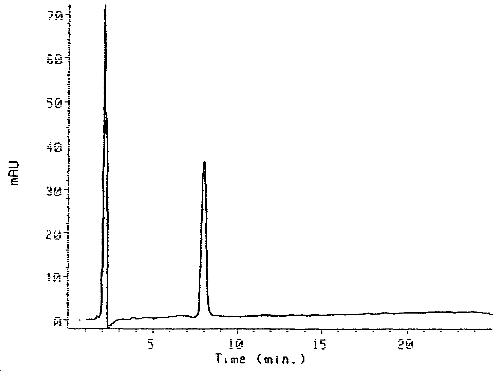
Figure 2. Chromatogram of the Purified HDI Biuret Derivative
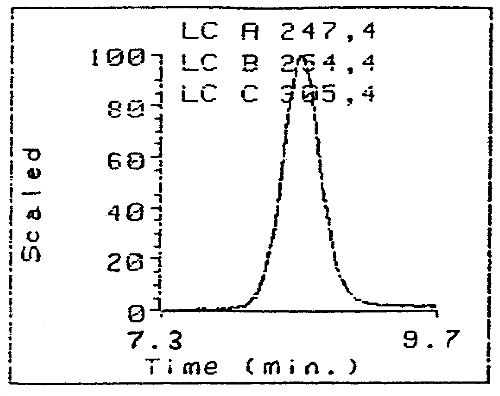
Figure 3. Three Wavelengths Monitoring on a Diode Array Detector of
the Purity of the Purified HDI Biuret Derivative
(Note the three
traces superimpose perfectly indicating high purity.)
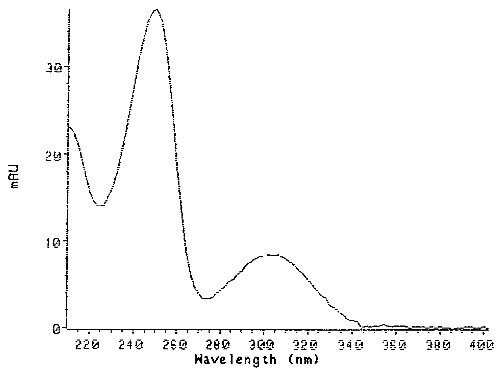
Figure 4. UV Scan of the Pure HDI Biuret Derivative
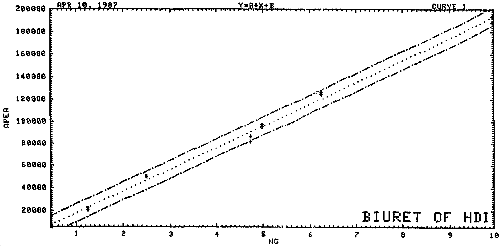
Figure 5. Calibration Curve of HDI Biuret
5. References
5.1. Hervin, R.L. and T.W. Thoburn, "Health Hazard Evaluation
Report 72-96-237: Trans World Airlines Main Overhaul Facility, Kansas
City International Airport", Hazard Evaluation Branch, NIOSH,
Cincinnati, Ohio, Report No.
5.2. Rosenberg, C. and T. Tuomi, "Airborne Isocyanates in
Polyurethane Spray Painting: Determination and Respiratory
Efficiency", ACGIH Journal, 1984, Vol. 45, No. 2, pp.
5.3. Stephenson, R.L., T.C. Aw, and D. O'Brien, "Health Hazard
Evaluation Report, No.
5.4. Grayson, M., ed., "Kirk-Othmer Encyclopedia of Chemical Technology", Third Ed., Vol. 13, p. 807. Interscience Publishers, New York, N.Y., 1978.
5.5. "Industrial Hygiene Technical Manual", OSHA Instruction CPL 2-2.20A, March 30, 1984, U.S. Department of Labor. Chapter II: "Standard Methods for Sampling Air Contaminants."
5.6. Goldberg, P.A., R.F. Walker, P.A. Ellwood; J.
Chromatogr. 1981, 212,