ISOPHORONE DIISOCYANATE (IPDI)
| Method number: | PV2034 |
| Matrix: | Air |
| Target Concentration: | 0.045 mg/m3 or 0.005 ppm (ACGIH TLV). |
| Procedure: | Samples are collected by drawing known volumes of air through open-faced cassettes containing glass fiber filters coated with 1.0 mg of 1-(2-pyridyl)piperazine (l-2PP). Samples are extracted with 90/10 (V/V) acetonitrile/dimethyl sulfoxide (ACN/DMSO) and analyzed by high performance liquid chromatography (HPLC) using an ultraviolet (UV) or fluorescence detector. |
| Recommended air volume and sampling rate: | 60 L at 1 L/min |
| Detection limit of the overall procedure (based on the recommended air volume): | 0.3 µg/m3 |
| Special requirements: | If the coated glass fiber filters are to be stored for any length of time before sampling, they should be kept in a refrigerator. |
| Status of method: | Stopgap method. This method has been only partially evaluated and is presented for in formation and trial use. |
| Date: April, 1988 (final) | Chemist: David B. Armitage |
Carcinogen and Pesticide Branch
OSHA Analytical
Laboratory
Salt Lake City, Utah
1. General Discussion :
- 1.1. Background
- 1.1.1. History of procedure
This evaluation was undertaken to determine the effectiveness of a l-2PP coated glass fiber filter as a sampling device for IPDI. It follows the procedure developed for methylene bisphenyl isocyanate (MDI). (Ref. 5.1.)
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy).
IPDI has been reported to produce skin sensitization in both humans and animals. (Ref. 5.2.)
IPDI has been found to provoke allergic dermatitis. Four sensitized workers were patch tested with IPDI. Tests were found to be strongly positive. None of the control subjects were positive. (Ref. 5.2.)
IPDI has exhibited severe respiratory problems in spray painters exposed to paints containing IPDI. (Ref. 5.2.)
From the available information on IPDI it appears that its toxicological action is similar to that of toluene-2,4-diisocyanate (TDI). For this reason the ACGIH adopted a TLV of 0.005 ppm (0.045 mg/m3, the same as TDI . (Ref. 5.2.)
1.1.3. Potential workplace exposure
No estimate of worker exposure to IPDI could be found.
IPDI yields high stability polyurethanes which are exceptionally resistant to discoloration by light and have great chemical resistance. It is used in applications where such a high quality polyurethane is desirable (i.e. car paints). It is also used as an elastomer. (Ref. 5.2.)
1.1.4. Physical properties (Ref. 5.2.)
| Molecular weight: | 222.3 |
| Molecular formula: | C12H18N2O2 |
| CAS #: | 4098-71-9 |
| Boiling point: | 158 #176;C at 10 mm Hg |
| Helting point: | approx. -60.°C |
| Density: | 0.90 at 20° C |
| Appearance: | colorless to slightly yellow liquid |
| Solubility: | completely miscible with esters, ketones, ethers, and aromatic and aliphatic hydrocarbons. |
| Synonyms: | IPDI; 5-isocyanato-l-(isocyanato-methyl)-1,3,3 trimethylcyclohexane; isophorone diisocyanate. |
| Chemical name: | 3-isocyanatomethyl-3,5,5-trimetyl cylcohexylisocyanate |
| Structure: | 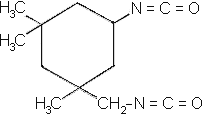 |
(consists of two isomers, which elute at different times)
1.2. Limit defining parameters
The detection limit of the analytical procedure is 0.043 ng per injection. This is the amount of analyte which will give a peak whose height is approximately five times the baseline noise.
2. Sampling procedure
2.1. Apparatus
2.1.1. A personal sampling pump that can be calibrated to within ± 5% of the recommended flow rate with the sampling device in line.
2.1.2. A three-piece styrene cassette containing a glass fiber filter coated with 1.0 mg of l-2PP and an untreated backup pad (See Figure 1.).
2.2. Glass fiber filter coating procedure
Prepare a solution of 2 mg/mL of l-2PP in methylene chloride. Spike the glass fiber filters with 0.5 mL of this solution. Allow the filters to air dry, and then dry them in a 40 °C vacuum oven for an additional 2 hours.
Store coated glass fiber filters in a closed jar at reduced temperature as a precaution to prevent decomposition of the l-2PP. Avoid exposure to strong sunlight.
2.3. Sampling technique
2.3.1. Perform open-faced sampling by removing the top cover (with small plug in place) from the three-piece cassette and the small plug from the exit port.
2.3.2. Attach the cassette to the sampling pump with flexible, plastic tubing such that the open face is vertically downward in the worker's breathing zone and in such a manner that it does not impede work performance.
2.3.3. After sampling for the appropriate time, remove the sampling device and replace the small exit plug and the top cover.
2.3.4. Wrap each sample end-to-end with an OSHA seal (Form 21).
2.3.5. Submit at least one blank with each set of samples. Handle the blanks the same as the other samples except draw no air through them.
2.3.6. Submit any bulk samples in a separate container. Do not ship them with the air samples.
2.4. Extraction efficiency
Nine l-2PP coated glass fiber filters were each liquid spiked with 8 µL of a DMSO solution of IPDI l-2PP derivative equivalent to 325 µg/mL IPDI. Three of these filters, along with a blank filter, were extracted with 4.0 mL 90/10 (V/V) ACN/DMSO and then analyzed. The remaining six filters were used in the retention and storage studies.
Glass Fiber Filter Extraction Study
| Filter # | Amount Spiked | Amount Recovered | % Recovery |
| E1 | 2.60 µg | 2.523 µg | 97.0 |
| E2 | 2.60 µg | 2.604 µg | 100.2 |
| E3 | 2.60 µg | 2.582 µg | 99.3 |
| BL | 0.00 µg | 0.00 µg | Blank |
|
| |||
2.5. Retention efficiency
The remaining six spiked filters each had 60 liters of humid air (65% relative humidity) drawn through them. Three of these filters were extracted with 4.0 mL 90/10 (V/V) ACN/DMSO and analyzed immediately. The remaining three filters were stored at room temperature in a drawer for the storage study.
Retention Efficiency Study
| Tube # | Amount spiked | Amount recovered | % Recovery |
| Rl | 2.60 µg | 2.531 µg | 97.3 |
| R2 | 2.60 µg | 2.539 µg | 97.7 |
| R3 | 2.60 µg | 2.500 µg | 96.2 |
|
| |||
2.6. Sample storage
The remaining three spiked filters were stored for a total of 7 days in a drawer at room temperature. They were then extracted with 4.0 mL 90/10 (V/V) ACN/DMSO and analyzed.
Storage Study
| Tube # | Amount spiked | Amount recovered | % Recovery |
| ST 1 | 2.60 µg | 2.478 µg | 95.3 |
| ST 2 | 2.60 µg | 2.574 µg | 99.0 |
| ST 3 | 2.60 µg | 2.520 µg | 96.9 |
|
| |||
2.7. Recommended air volume and sampling rate.
2.7.1. The recommended air volume is 60 L.
2.7.2. The recommended flow rate is 1 L/min.
2.8. Interferences
Compounds such as acid chlorides; anhydrides, and other isocya nates that react with the l-2PP may compete for the derivatizing agent on the glass fiber filter and diminish the latter's effectiveness.
Suspected interferences should be reported to the laboratory with submitted samples.
2.9. Safety precautions
2.9.1. Attach the sampling equipment in such a manner that it will not interfere with work performance or employee safety.
2.9.2. Follow all safety practices that apply to the work area being sampled.
3. Analytical procedure
- 3.1. Apparatus
3.1.1. A high performance liquid chromatograph equipped with a UV or fluorescence detector, and manual or automatic injector. A Waters M6000A pump, Waters 712 autosampler, Kratos Spectroflow 980 fluorescence detector, and a Waters 440 dual wavelength UV detector were used in this evaluation.
3.1.2. An HPLC column capable of separating IPDI from any interferences. A 25 cm x 4.6 mm i.d. Chromegabond 5 micron TMS column was used in this evaluation.
3.1.3. An electronic integrator or other suitable means of measuring detector response. A Hewlett-Packard 3357 data system was used in this evaluation.
3.1.4. Vials, 4-mL glass with polytetrafluoroethylene (PTFE)-lined septa.
3.1.5. Volumetric flasks, pipets, and syringes for preparing standards, making dilutions, and performing injections.
3.2.1. HPLC grade ACN.
3.2.2. HPLC grade water. A Millipore Milli-Q system was used to prepare the water in this evaluation.
3.2.3. HPLC grade DMSO.
3.2.4. IPDI l-2PP derivative prepared by D. Burright.
3.3. Standard preparation
Prepare stock standard solutions by adding DMSO to preweighed amounts of IPDI l-2PP derivative. Prepare working range standard solutions by diluting stock solutions with 90/10 (V/V) ACN/DMSO. Apply a correction factor of 0.405 (F.W. of IPDI, 222.3 / F.W. of the l-2PP derivative, 548.89) to express concentrations in terms of IPDI. Store stock and dilute standards in a freezer.
3.4. Sample preparation
3.4.1. Open the styrene cassette and place the coated glass fiber filter in a 4-mL vial so that the filter is flat against the inside of the vial. Do not fold or crumple it.
3.4.2. Add 4.0 mL of 90/10 (V/V) ACN/DMSO to each vial.
3.4.3. Seal the vials with PTFE-lined septa and allow them to extract for one hour. Shake the vials by hand periodically during this extraction time.
3.5. Analysis
3.5.1. Instrument conditions
| Column: | 25 cm x 4.6 mm i.d. Chromegabond 5 micron TMS |
| Mobile Phase: | 49.5% ACN/50.5% water, with 0.045 M ammonium acetate buffer adjusted to pH 5.9 |
| Flow rate: | 1 ml/minute |
| Fluorescence Detector: | excitation = 240 nm |
| emission = 370 nm | |
| UV Dectector: | 254 nm |
| Retention times: | 7.3 and 9.0 minutes |
| Injection volume: | 10 µL |
3.5.2. Chromatogram (See Figure 2)
3.6. Interferences
3.6.1. Any compound having a similar retention time to the analyte is a potential interference. Generally, chromategraphic conditions can be altered to separate an interference from the analyte.
3.6.2. Retention time on a single column is not proof of chemical identity. Analysis by an alternate HPLC column, detection at another wavelength, comparison of absorbance response ratios (or comparison of UV to fluorescence response ratios), and confirmation by mass spectrometry are additional means of identification.
3.7. Calculations
3.7.1. A calibration curve is constructed by plotting detector response versus standard concentration.
3.7.2. The concentration of IPDI in a sample is determined from the calibration curve.
3.7.3. The total IPDI concentration is then determined by the following formula.
| mg/m3 = | (µg/mL, blank corrected) ×
(desorption volume, mL)
(air volume, L) × (desorption efficiency, decimal) |
3.8. Safety precautions
3.8.1. Avoid exposure to all standards.
3.8.2. Avoid exposure to all solvents.
3.8.3. Wear safety glasses at all times.
4. Recommendations for further study
This method should be fully validated.
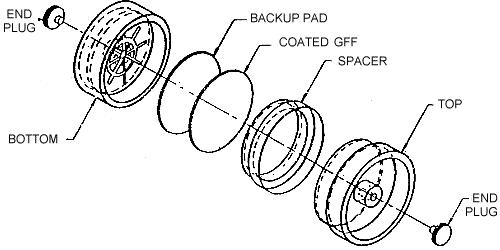
Figure 1. Sample Cassette
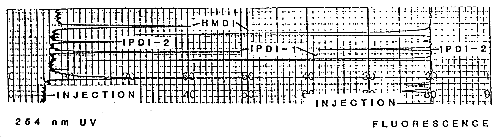
Figure 2. Chromatogram of IPDI (with HMDI)
5. References
5.1. "OSHA Analytical Hethods Manual", U.S. Department of Labor,
Occupational Safety and Health Administration, OSHA Analytical
T,aboratory: Salt Lake City, UT, Method 47, American Conference of
Governmental Industrial Hygienists (ACGIH): Cincinnati, OH, 1985,
ISBN:
5.2. "Documentation of the Threshold Limit Values and Biological Exposure Indices", American Conference of Governmental Industrial Hygienists Inc., fifth edition, 1986.