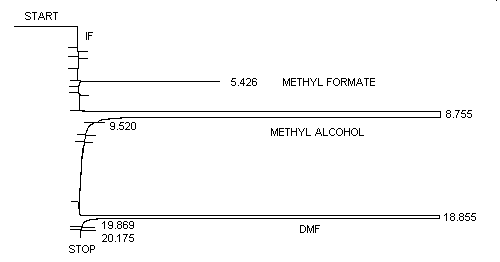
METHYL FORMATE
| Method number: | PV2041 |
| Matrix: | Air |
| Target Concentration: | 100 ppm (246 mg/m3) OSHA permissible exposure limit (PEL). |
| Procedure: | Samples are collected by drawing known volumes of air through Anasorb 747 sampling tube (6-mm i.d. glass tube, the front section contains 400 mg and the back 200 mg of sorbent). Samples are desorbed with a 90:10 (v/v) methyl alcohol/dimethylformamide solution and analyzed by gas chromatography (GC) using a flame ionization detector (FID). |
| Recommended air volume and sampling rate: |
3 L at 0.05 L/min |
| Detection limit of the overall procedure (based on the recommended air volume and the analytical detection limit): | 1.16 ppm (2.84 mg/m3) |
| Special requirements: | Ship the samples cold after sampling to the laboratory and analyzed immediately. |
| Status of method: | Partially evaluated method. This method has been partially evaluated and is presented for information and trial use only. |
| April 1992 (final) | Chemist: |
Organic Service Branch II
OSHA Technical Center
Salt
Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History of procedure
This evaluation was undertaken to develop a sampling and analytical procedure for methyl formate at the OSHA PEL 100 ppm (Ref. 5.1.).
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
Inhalation of vapor produces nasal and conjunctival irritation, retching, narcosis, and death from pulmonary effects (Ref. 5.2., 5.3. and 5.4.).
1.1.3. Potential workplace exposure
Methyl formate has been employed as a fumigant and larvicide, as well as a solvent for cellulose acetate and in organic synthesis. (Ref. 5.2., 5.3. and 5.4.). No data is available on the extent of work place exposure.
1.1.4. Physical properties (Ref. 5.2., 5.3. and 5.4.)
| CAS number: | 107-31-3 |
| IMIS number: | 1770 |
| Molecular weight: | 60.05 |
| Molecular formula: | C2H4O2 |
| Density: | 0.987 at 20°C |
| Boiling point: | 31.5°C at 101.3 kPa (760 mmHg) |
| Solubility: | soluble in about 3.3 parts water miscible with alcohol and ether |
| Chemical name: | methyl formate |
| Synonyms: | methyl methanoate formic acid methyl ester |
| Appearance: | colorless liquid with an agreeable odor |
| Structure: | HCOOCH3 |
1.2. Limit defining parameters
The detection limit of the analytical procedure, including a 2.7:1 split ratio, is 3.16 ng per injection. This is the amount of analyte which will give a peak whose height is approximately five times the baseline noise.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. A sample is collected by using a personal sampling pump
that can be calibrated to within ± 5% of the recommended flow rate
with the sampling device in line.
2.1.2. A sample is collected with
2.2. Reagents
No sampling reagents are required.
2.3. Sampling technique
- 2.3.1. Immediately before sampling, break off the ends of the
sampling tube. All tubes should be from the same lot.
2.3.2. Attach the sampling tube to the sampling pump with
flexible tubing. Position the tube so that sampled air first passes
through the
2.3.3. Attach the tube vertically in the employee's breathing zone in such a manner that it does not impede work performance.
2.3.4. After sampling for the appropriate time, remove the sample tube and seal it with plastic caps.
2.3.5. Wrap each sample
2.3.6. Record the air volume for each sample and list any possible interferences.
2.3.7. Submit at least one blank for each set of samples. Handle the blank in the same manner as the samples, except no air is drawn through it.
2.3.8. Ship the samples cold after sampling to the laboratory and analyzed immediately.
2.3.9. Submit bulk samples for analysis in a separate container. Do not ship bulk samples with air samples.
2.4. Desorption efficiency
Twelve vials, each containing
Desorption Efficiency
|
| |||
Sample # |
Amount Spiked, µg |
Amount Found, µg |
% Recovered |
| D1 D2 D3 |
115 115 115 |
115 112 115 |
100.0 97.4 100.0 |
| Average of 0.15× PEL = 99.1% | |||
| D4 D5 D6 |
366 366 366 |
343 343 347 |
93.7 93.7 94.8 |
| Average of 0.5× PEL = 94.0% | |||
| D7 D8 D9 |
770 770 770 |
717 729 742 |
93.1 94.7 96.4 |
| Average of 1× PEL = 94.7% | |||
| D10 D11 D12 D13 |
1540 1540 1540 Blank |
1427 1474 1494 0 |
92.7 95.7 97.0 0.0 |
| Average of 2× PEL = 95.1% | |||
|
| |||
2.5. Retention efficiency
Four Anasorb 747 tubes were each liquid spiked with 1.6 µL (1× PEL) of 50% methyl formate in methyl alcohol. These were allowed to equilibrate for 2 hours and then 3 L of humid air (~80% relative humidity) were drawn through each tube at 0.05 L/min. Then the tubes were desorbed with 3.0 mL of desorbing solution, and then analyzed as in Section 3. The results are listed in Table 2.5.
Retention Efficiency
|
| |||
Sample # |
Amount Spiked, µg |
Amount Found, µg |
% Recovered |
| R1 R2 R3 R4 |
770 770 770 770 |
711 724 770 748 |
92.3 94.0 100.0 97.1 |
| Average = 95.9% | |||
|
| |||
2.6. Sample storage
Nine Anasorb 747 tubes were each liquid spiked with 1.6 µL (1× PEL) of 50% methyl formate in methyl alcohol. These were allowed to equilibrate for 2 hours and then 3 L of humid air (~80% relative humidity) were drawn through each tube at 0.05 L/min. The nine tubes were divided into three groups of three tubes each. The first group was stored in a drawer at ambient temperature, the second group was stored in a refrigerator (0°C) and the third group was stored in a freezer (-5°C). After seven days they were extracted and analyzed as in Section 3. No analytes were observed in backup section. The results are given in Tables 2.6.1., 2.6.2. and 2.6.3..
Ambient Storage
|
| |||
| Days Stored |
Amount Spiked, µg |
Amount Found, µg |
% Recovered |
| 7 7 7 |
770 770 770 |
95 143 91 |
12.3 18.6 11.8 |
| Average = 14.2% | |||
|
| |||
Refrigerator Storage
|
| |||
| Days Stored |
Amount Spiked, µg |
Amount Found, µg |
% Recovered |
| 7 7 7 |
770 770 770 |
615 640 633 |
79.9 83.1 82.2 |
| Average = 81.7% | |||
|
| |||
Freezer Storage
|
| |||
| Days Stored |
Amount Spiked, µg |
Amount Found, µg |
% Recovered |
| 7 7 7 |
770 770 770 |
623 642 649 |
80.9 83.4 84.3 |
| Average = 82.9% | |||
|
| |||
2.7. Recommended air volume and sampling rate
- 2.7.1. The recommended air volume is 3 L.
2.7.2. The recommended flow rate is 0.05 L/min.
2.8. Interferences (sampling)
It is not known if any compounds will interfere with the collection of methyl formate. Any suspected interferences should be reported to the laboratory with submitted samples.
2.9. Safety precautions (sampling)
- 2.9.1. Attach the sampling equipment in such a manner that it
will not interfere with work performance or employee safety.
2.9.2. Follow all safety practices that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A GC equipped with an FID. A
3.1.2. A GC column capable of separating methyl formate from any interferences. A 60 m × 0.32 mm i.d. (1.0 µm film) STABILWAX capillary column was used in this evaluation.
3.1.3. An electronic integrator or some other suitable means to measure detector response. A Waters 860 Networking Computer System was used in this evaluation.
3.1.4. Volumetric flasks, pipets, and syringes for preparing standards, making dilutions and performing injections.
3.1.5. Vials,
3.1.6. Mechanical shaker.
3.2. Reagents
- 3.2.1. Methyl formate. Methyl formate, 97.5+% purity, was
obtained from Eastman Chemical Company.
3.2.2. Methyl alcohol. The methyl alcohol used in this evaluation was purchased from Fisher Scientific.
3.2.3. Dimethylformamide (DMF). The DMF was purchased from Burdick and Jackson.
3.2.4. Desorbing solution, 90/10 (v/v) methyl alcohol and DMF.
3.3. Standard preparation
Prepare standards at concentrations of 1 µL, 2 µL and 4 µL of methyl formate per milliliter of desorbing solution. Standards must be used the day they are prepared.
3.4. Sample preparation
- 3.4.1. Transfer the
3.4.2. Add 3.0 mL of desorbing solultion to each vial and seal
with a
3.4.3. Shake the vials on a mechanical shaker for an hour.
3.5. Analysis
- 3.5.1. Instrument conditions
| Column: | STABILWAX, 60 m × 0.32 mm i.d., 1.0 µm film |
| Injector temperature: | 150°C |
| Detector temperature: | 200°C |
| Column temperature: | 50°C (initial temp) |
| Temperature program: | hold initial temp. 5 min, increase temp. at
10°C/min to |
| Gas flow rates: | |
| column: septum purge: FID: FID: FID: |
2.0 mL/min (hydrogen) 7.5 mL/min (hydrogen) 32 mL/min (hydrogen) 34 mL/min (nitrogen) 400 mL/min (air) |
| Injection volume: | 1 µL |
| Split ratio: | 2.7:1 |
| Retention time: | 5.4 min (methyl formate) 8.7 min (methyl alcohol) 18.9 min (DMF) |
3.5.2. Chromatogram (Figure 1.)
3.5.3. Measure detector response using a suitable method such as electronic integration.
3.6. Interferences (analytical)
- 3.6.1. Any collected compound which produces an FID response and
has a similar retention time as methyl formate is a potential
interference.
3.6.2. GC conditions may generally be varied to circumvent interferences.
3.6.3. Retention time on a single column is not proof of chemical identity. Analysis by an alternate GC column, high performance liquid chromatography (HPLC) and confirmation by mass spectrometry are additional means of identification.
3.7. Calculations
- 3.7.1. An external standard (ESTD) calibration method is used. A
calibration curve may be constructed by plotting concentration of
analyte per sample versus response of standard concentration (µg/mL)
of methyl formate. Bracket the samples with freshly prepared
analytical standards over a range of concentrations.
3.7.2. Determine the µg/mL of methyl formate in both sections of each sample and blank from the calibration curve. If methyl formate is found on the backup section, it is added to the amount found on the front section. Blank corrections should be performed before adding the results together.
3.7.3. Determine the air concentration by using the following formula.
| mg/m3 = | (µg/mL, blank corrected) ×
(desorption volume, mL)
(air volume, L) × (desorption efficiency, decimal) |
| ppm = | (mg/m)(24.46)
(60.05) |
| where | 24.46 60.05 |
= = |
molar volume (liters) at 101.3 kPa (760 mmHg)
and 25°C molecular weight of methyl formate |
3.8. Safety precautions (analytical)
- 3.8.1. Avoid skin contact and air exposure to methyl formate.
3.8.2. Avoid skin contact with all solvents.
3.8.3. Wear safety glasses in laboratory.
4. Recommendation for Further Study
This method should be fully validated.
5. References
- 5.1. "Code of Federal Regulations", 29 CFR
1910.1000, Table Z-1-A. Limits for Air Contaminants, U.S. Government
Printing Office, Washington, D.C., 1990.
5.2. Documentation of the Threshold Limit Values and Biological Exposure Indices, American Conference of Governmental Industrial Hygienist INC., 5th ed., 1986; p 397.
5.3. Sitting, M., Handbook of Toxic and Hazardous Chemicals, Noyes Publications, Park Ridge, N.J., 1981; p 453.
5.4. Windholz, M., Budavari, S., Blumetti, RF., and Otterbein, E., The Merck Index, 10th ed., Merck & CO., Inc., Rahway, N.J., 1983; p 870.