N-METHYL-2-PYRROLIDINONE
| Method number: | PV2043 |
| Matrix: | Air |
| Target concentration: | 51 ppm (103 mg/m3) |
| Procedure: | Samples are collected by drawing a known volume of air through a charcoal tube. Samples are desorbed with 95:5 methylene chloride:methanol and analyzed by gas chromatography with a flame ionization detector (GC-FID). |
| Air volume and sampling rate studied: |
10 liters at 0.2 Lpm. |
| Status of method: | Stopgap method. This method has been only partially evaluated and is presented for information and trial use. |
| Date: June, 1991 | Chemist: Mary E. Eide |
Organic Service Branch I
OSHA Technical Center
Salt
Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History of procedure
The OSHA Technical Center has received many requests for a sampling and analytical procedure for methyl pyrrolidinone. Charcoal tube sampling and desorption with 1 mL carbon disulfide was tried initially, but the desorption efficiency averaged 32.7%. Desorption with 1 mL 99:1 carbon disulfide:DMF had concentration dependent desorption ranging from 73.2 to 84.4 for concentrations ranging from 2.066 to 16.528 mg. Desorption with 1 mL 95:5 methylene chloride:methanol averaged 99.4% for concentrations from 0.1033 to 2.066 mg. Charcoal tubes had good storage and retention efficiencies. A target level of 51 ppm was chosen as it is half the recommended TWA TLV listed on MSDS from several manufacturers.
1.1.2. Potential workplace exposure (Ref. 5.1.)
Methyl pyrrolidinone is used as a solvent in many applications. It is used as a solvent to facilitate the formation of many chemicals. It is used as a solvent for various extractions in cracking oils and in further petrochemical reactions. It is used as a medium for polymerization, and as a solvent for finished polymers. It is used in stripping potting resins and epoxides.
1.1.3. Toxic Effects (This section is for information purposes and should not be taken as the basis for OSHA policy.)(Ref. 5.2.)
Methyl pyrrolidinone in rats had a TDLo of 7500 mg/kg for skin exposure and a LD50 of 3564 mg/kg, with weight loss and body temperature decreases noted in the survivors. In humans, methyl pyrrolidinone is an irritant to eyes, skin and mucous membranes. Prolonged exposure may cause headache, nausea, vomiting and dizziness.
1.1.4. Physical properties (Ref. 5.3.):
| Compound: |  |
| Synonyms: | 1-Methyl-2-pyrrolidinone;
|
| Molecular weight: | 99.13 |
| Density: | 1.033 |
| Freezing point: | -24°C |
| Boiling point: | 202°C |
| Flash point: | 95°C (204°F) (open cup) |
| Color: | colorless liquid |
| Odor: | mild amine-like |
| Molecular formula: | C5H9NO |
| CAS: | 872-50-4 |
| IMIS: | M139 |
| RTECS: | 74457 (UY5790000) |
1.2. Limit defining parameters
1.2.1. The detection limit of the analytical procedure is 4 ng with a 1 µL injection volume. This is the smallest amount which could be detected under normal operating conditions.
1.2.2. The overall detection limit is 0.1 ppm based on a 10 liter air volume. (All ppm amounts in this study are based on a 10 L air volume.)
1.3. Advantages
1.3.1. The sampling procedure is convenient.
1.3.2. The analytical method is reproducible and sensitive.
1.3.3. Reanalysis of samples is possible.
1.3.4. It may be possible to analyze other compounds at the same time.
1.3.5. Interferences may be avoided by proper selection of column and GC parameters.
1.4. Disadvantages
Due to the volatility of the methylene chloride in the desorbing solvent, it may be necessary to have a fan blowing on the instrument, in order to have consistent injections, when using an autosampler.
2. Sampling procedure
2.1. Apparatus
2.1.1. A calibrated personal sampling pump, the flow of which can be determined within ± 5% at the recommended flow.
2.1.2. Charcoal tubes, lot 120, containing 100 mg adsorbing section with a 50 mg backup section separated by a 2 mm portion of urethane foam, with a silanized glass wool plug before the adsorbing section and a 3 mm plug of urethane foam at the back of the backup section. The ends are flame sealed and the glass tube containing the adsorbent is 7 cm long, with a 6 mm O.D. and 4 mm I.D., SKC tubes or equivalent.
2.2. Sampling technique
2.2.1. Open the ends of the charcoal tubes immediately before sampling.
2.2.2. Connect the charcoal tube to the sampling pump with flexible tubing.
2.2.3. Place the tubes in a vertical position to minimize channeling, with the smaller section towards the pump.
2.2.4. Air being sampled should not pass through any hose or tubing before entering the charcoal tube.
2.2.5. Seal the charcoal tube with plastic caps immediately after
sampling. Seal each sample lengthwise with OSHA
2.2.6. With each batch of samples, submit at least one blank tube from the same lot used for samples. This tube should be subjected to exactly the same handling as the samples (break ends, seal, & transport) except that no air is drawn through it.
2.2.7. Transport the samples (and corresponding paperwork) to the lab for analysis.
2.2.8. Bulks submitted for analysis must be shipped in a separate mailing container from other samples.
2.3. Desorption efficiency
Six tubes were liquid spiked at each loading of 0.103, 0.517, 1.03, and 2.07 mg/mL methyl pyrrolidinone. They were allowed to equilibrate overnight at room temperature. They were opened, each section placed into a separate 2 mL vial, desorbed with 1 mL of the desorbing solution, desorbed for 30 minutes with occasional shaking, and were analyzed by GC-FID. The overall average was 99.4 %.(Table 1)
Table 1
Desorption Efficiency
|
| ||||
| Tube# | % Recovered | |||
| 0.103 mg | 0.517 mg | 1.03 mg | 2.07 mg | |
|
| ||||
| 1 | 99.9 | 99.5 | 100 | 100 |
| 2 | 98.8 | 100 | 96.9 | 99.6 |
| 3 | 98.5 | 99.8 | 100 | 99.6 |
| 4 | 99.1 | 100 | 100 | 99.7 |
| 5 | 98.2 | 99.5 | 99.0 | 100 |
| 6 | 99.0 | 99.8 | 99.3 | 99.7 |
| average |
99.0 |
99.8 |
99.3 |
99.7 |
| overall average | 99.4 | |||
| standard deviation | ± 0.735 | |||
|
| ||||
2.4. Retention efficiency
Six tubes were liquid spiked with 2.07 mg (51.0 ppm) methyl pyrrolidinone, allowed to equilibrate overnight, and had 10 liters humid air (91% RH) pulled through them. They were opened, desorbed and analyzed by GC-FID. The retention efficiency averaged 99.6%. There was no methyl pyrrolidinone found on the backup portions of the tubes. (Table 2)
Table 2
Retention Efficiency
|
| ||||
| Tube # | % Recovered | % Recovered | Total | |
| 'A' | 'B' | |||
|
| ||||
| 1 | 99.9 | 0.0 | 99.9 | |
| 2 | 97.7 | 0.0 | 97.7 | |
| 3 | 98.9 | 0.0 | 98.9 | |
| 4 | 99.9 | 0.0 | 99.9 | |
| 5 | 101 | 0.0 | 101 | |
| 6 | 100 | 0.0 | 100 | |
| average | 99.6 | |||
|
| ||||
2.5. Storage
Tubes,were spiked with 2.07 mg (51.0 ppm) methyl pyrrolidinone and stored at room temperature until opened and analyzed. The recoveries averaged 99.3 % for the 15 days stored.(Table 3)
Table 3
Storage Study
|
| |||
| Day | % Recovered | ||
|
| |||
| 7 | 101 | ||
| 7 | 97.0 | ||
| 7 | 95.9 | ||
| 15 | 100 | ||
| 15 | 101 | ||
| 15 | 101 | ||
| average | 99.3 | ||
|
| |||
2.6. Precision
The precision was calculated using the area counts from six injections of each standard at concentrations of 0.103, 0.517, 1.03, and 2.07 mg/mL methyl pyrrolidinone in the desorbing solvent. The pooled coefficient of variation was 0.00731.(Table 4)
Table 4
Precision Study
|
| |||||
| Injection | |||||
| Number | 0.103 mg/mL | 0.517 mg/mL | 1.03 mg/mL | 2.07 mg/mL | |
|
| |||||
| 1 | 31014 | 147390 | 286310 | 568130 | |
| 2 | 31413 | 147880 | 283730 | 566190 | |
| 3 | 31606 | 146470 | 286010 | 571550 | |
| 4 | 31033 | 147830 | 288630 | 572980 | |
| 5 | 31339 | 147000 | 288270 | 565930 | |
| 6 | 31196 | 145030 | 282800 | 574330 | |
| Average | 31267 | 146933 | 285958 | 569852 | |
| Standard | |||||
| Deviation | ± 230 | 1072 | 2347 | 3591 | |
| CV | 0.00736 | 0.00730 | 0.00821 | 0.00630 | |
| Pooled CV | 0.00731 | ||||
|
| |||||
where:

A(1), A(2),A(3),A(4) = # of injections at each
level
CVl, CV2, CV3, CV4 = Coefficients at each level
2.7. Air volume and sampling rate studied
2.7.1. The air volume studied is 10 liters.
2.7.2. The sampling rate studied is 0.2 liters per minute.
2.8. Interferences
Suspected interferences should be listed on sample data sheets.
2.9. Safety precautions
2.9.1. Sampling equipment should be placed on an employee in a manner that does not interfere with work performance or safety.
2.9.2. Safety glasses should be worn at all times.
2.9.3. Follow all safety practices that apply to the workplace being sampled.
3. Analytical method
3.1. Apparatus
3.1.1. Gas chromatograph equipped with a flame ionization detector. A HP 5890 was used in this study.
3.1.2. GC column capable of separating the analyte and an
internal standard from any interferences. The column used in this
study was a 60 meter
3.1.3. An electronic integrator or some other suitable method of measuring peak areas.
3.1.4. Two milliliter vials with Teflon-lined caps.
3.1.5. A 10 µL syringe or other convenient size for sample injection.
3.1.6. Pipets for dispensing the desorbing solution. The Glenco 1 mL dispenser was used in this method.
3.1.7. Volumetric flasks - 5 mL and other convenient sizes for preparing standards.
3.2 Reagents
3.2.1. Purified GC grade nitrogen, hydrogen, and air.
3.2.2. Methyl pyrrolidinone, Reagent grade
3.2.3. Methylene chloride, HPLC grade
3.2.4. Methanol, HPLC grade
3.2.5. n-Hexanol, Reagent grade, used as the internal standard
3.2.6. The desorbing solution is 95:5 methylene chloride:methanol
with 0.25 µL/mL
3.3. Sample preparation
3.3.1. Sample tubes are opened and the front and back section of each tube are placed in separate 2 mL vials.
3.3.2. Each section is desorbed with 1 mL of the desorbing solution of 95:5 methylene chloride:methanol with 0.25 µL/mL n-hexanol internal standard.
3.3.3. The vials are sealed immediately and allowed to desorb for 30 minutes with occasional shaking.
3.4. Standard preparation
3.4.1. Standards are prepared by diluting a known quantity of methyl pyrrolidinone with the desorbing solution. A standard of 1 µL/mL methyl pyrrolidinone in the desorbing solution is 1033 µg/mL.
3.4.2. At least two separate standards at the calibration level should be made.
3.4.3. A third analytical standard should be prepared at a higher concentration to check the linearity of the detection. For this study two standards at 1 µL/mL (1.033 mg/mL) and one standard at 4 µL/mL (4.132 mg/mL) methyl pyrrolidinone were used.
3.5. Analysis
3.5.1. Gas chromatograph conditions.
| Flow rates (mL/min.) | Temperature (°C) | ||
| Nitrogen (make-up): | 30 | Injector: | 200 |
| Hydrogen (carrier): | 2 | Detector: | 225 |
| Hydrogen (detector): | 30 | Column: | 80°-1min |
| Air: | 350 | 10°/min-160° | |
| Injection size: | 1 µL | ||
| Chromatogram: | see Figure 1 |
3.5.2. Peak areas are measured by an integrator or other suitable means.
3.6. Interferences (analytical)
3.6.1. Any compound having the general retention time of the analyte or the internal standard used is an interference. Possible interferences should be listed on the sample data sheet. GC parameters should be adjusted if necessary so these interferences will pose no problems.
3.6.2. Retention time data on a single column is not considered proof of chemical identity. Samples over the target concentration should be confirmed by GC/Mass Spec or other suitable means.
3.7. Calculations
3.7.1. The instrument is calibrated with a standard of 1.033 mg/mL (1 µL/mL) methyl pyrrolidinone in the desorbing solution. The linearity of the calibration is checked with a standard of 4.132 mg/mL (4 µL/mL) methyl pyrrolidinone in the desorbing solution.
3.7.2. If the calibration is non-linear, two more standards must be analyzed so a calibration curve can be plotted and sample values obtained.
3.7.3. To calculate the concentration of analyte in the air sample the following formulas are used:
| (µg/m)(desorption volume)
(desorption efficiency) |
= mass of analyte in sample |
| (mass of analyte in sample)
molecular weight |
= number of moles of analyte |
| (number of moles of analyte) |
(molar volume at 25°C & 760mm) |
= | volume the analyte
will occupy at 25°C & 760mm |
| (volume analyte
occupies)(106)*
(air volume) |
= ppm |
3.7.4. The above equations can be consolidated to form the following formula. To calculate the ppm of analyte in the sample based on a 10 liter air sample:
| (µg/mL)(DV)(24.45)(106)
(100 L)(DE)(MW) |
× | (g)
(1000 mg) |
× | (mg)
(1000 µg) |
= ppm |
| µg/mL | = | concentration of analyte in sample or standard |
| 24.45 | = | Molar volume (liters/mole) at 25° and 760 mm Hg. |
| MW | = | Molecular weight (g/mole) |
| DV | = | Desorption volume |
| 10 L | = | 10 liter air sample |
| DE | = | Desorption efficiency |
3.7.5. This calculation is done for each section of the sampling tube and the results added together.
3.8. Safety precautions
3.8.1. All handling of solvents should be done in a hood.
3.8.2. Avoid skin contact with all solvents.
3.8.3. Wear safety glasses at all times.
4. Recommendations for further study
Collection studies need to be performed.
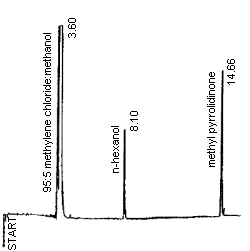
Figure 1. An analytical standard of 1.033 mg/mL methyl pyrrolidinone in
the desorbing solvent of 95:5 methylene chloride:methanol with n-hexanol
internal standard.
5. References
5.1. Grayson, M., "Kirk Othmer Encyclopedia of Chemical Technology", Third Edition, John Wiley & Son, N.Y., 1981, Vol. 19, p. 514.
5.2. Sweet, D., "Registry of Toxic Effects of Chemical Substances", 1985-86 Edition, U.S. Department of Health and Human Services, Public Health Service, Center for Disease Control, NIOSH, 1987, Vol. 5, p. 4221.
5.3. Sax, N., "Dangerous Properties of Industrial Materials", Fifth Edition, Van Nostrand Reinhold Co., New York, 1979, p. 831.