PARAFFIN WAX FUMES
| Method number: | PV2047 |
| Matrix: | Air |
| Target Concentration: | 2 mg/m3 ACGIH TWA TLV |
| Procedure: | Samples are collected by drawing a known volume of air through a glass fiber filter. Samples are desorbed with Carbon Disulfide and analyzed by gas chromatography using a Flame Ionization Detector. |
| Air volume and studied: | 100L at 1 Lpm. |
| Status of method: | Stopgap method. This method has been only partially evaluated and is presented for information and trial use. |
| Date: 6-9-88 | Chemist: Brett Besser |
SOLVENTS BRANCH
OSHA ANALYTICAL LABORATORY
SALT LAKE
CITY, UTAH
1. General Discussion
- 1.1. Background
- 1.1.1. History of procedure
The OSHA Laboratory recently received samples collected on glass fiber filters requesting paraffin wax fume analysis. Although this collection method has been recommended for many years, the supporting documentation had not been collected. Since there is a TLV and samples were received, it was decided to perform the laboratory work needed to evaluate this sampling and analytical procedure.
1.1.2. Toxicity
Pure paraffin wax is widely regarded as non toxic, but may possess some carcinogenic properties.(Ref 5.3) These properties are largely believed to be due to polycyclic aromatic hydrocarbons, but most processed waxes in use in America today do not possess any measurable levels of polycyclics. (Ref 5.1) Work around molten paraffin, especially if it is overheated is more uncomfortable and nauseating than dangerous. (Ref 5.4)
1.1.2. Potential workplace exposure
Workers are exposed to paraffin wax fumes in a variety of industries. Any time that paraffin solid is heated a fume may be produced. Paraffin is ideal for use as a sealer or waterproofing agent. Coating of paper for use as containers for milk is one of the largest uses of paraffin. (Ref 5.1) It is also used in the candle making industry and as an original mold in the casting industry.
1.1.3. Physical properties:
| Compound: | Paraffin Wax is macrocrystalline and is composed mostly, (40%-90%), of straight chain alkanes Cl8 - C36. The remainder is composed of Cl8 - C36 isoalkanes and cycloalkanes. Paraffin wax contains very little oil. (less than 0.1%) |
| Odor: | none |
| Color: | white |
| CAS: | 8002-74-2 |
| IMIS: | 2000 |
| RTECS: | RV0350000 |
1.2. Limit defining parameters
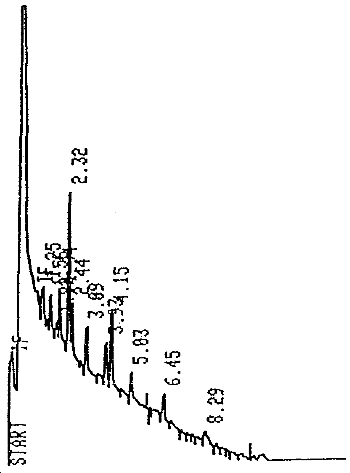
- 1.2.1. The detection limit of the analytical procedure is 6.8 ng
per injection. This is the smallest amount of paraffin that will
give a characteristic chromatographic pattern. (Figure 1)
1.2.2. The overall detection limit is 0.034 mg/m3 based on a 100 liter air volume and a 2 mL desorption volume. Air concentrations given throughout this procedure are based on a 100 liter air volume and 2 mL desorption volume.
1.3. Advantages
- 1.3.1. The sampling procedure is convenient.
1.3.2. The analytical method is reproducible and sensitive.
1.3.3. Reanalysis of samples is possible.
1.4. Disadvantages
- 1.4.1. Paraffin produces a characteristic fingerprint pattern.
Integration generally is done by using the total area of all peaks.
An interfering peak counted as part of the total area would produce
inaccurate results.
2. Sampling procedure
- 2.1. Apparatus
- 2.1.1. A calibrated personal sampling pump, the flow of which
can be determined within + 5% at the recommended flow.
2.1.2. A three piece plastic sampling cassette capable of holding a 37 mm glass fiber filter.
2.2. Sampling technique
- 2.2.1. The glass fiber filter is placed in the cassette and the
inlet and outlet plugs are removed.
2.2.2. Connect the sampling cassette to the sampling pump with flexible tubing.
2.2.3. Air being sampled should not pass through any hose or tubing before entering the sampling cassette.
2.2.4. Replace the inlet half of the cassette. Seal the cassette
with plastic plugs immediately after sampling. Seal each sample
covering the plugs with OSHA
2.2.6. With each batch of samples, submit at least one blank. This cassette should be subjected to exactly the same handling as the samples, except no air is drawn through it.
2.2.7. Send the samples and corresponding paperwork to the lab for analysis.
2.2.8. Bulks submitted for analysis must be shipped in a separate container from the air samples.
2.3. Extraction efficiency
- 2.3.1 Eighteen filters were spiked with a solution of Parowax in
carbon disulfide. The solution was 6.26 mg/mL. Six filters were
spiked with 6 uL (37 ug, six with 32 uL (200 ug) and the final six
with 120 uL (751 ug). This corresponds to approximately 0.1, 0.5 and
2 times the target concentration. The tubes were refrigerated
overnight, extracted the next day, and analyzed by gas
chromatography using a flame ionization detector. The average
extraction efficiency is 95.3%. (Table 1)
Extraction Efficiency
|
| ||||
| Sample | ||||
| Number | 2x | 0.5x | 0.1x | |
| 7.51 mg | 2.0 mg | 0.37 mg | ||
|
| ||||
| 1 | 95 | 98 | 99 | |
| 2 | 97 | 104 | 95 | |
| 3 | 92 | 96 | 91 | |
| 4 | 94 | 92 | 90 | |
| 5 | 102 | 102 | 86 | |
| 6 | 98 | 95 | 94 | |
| average | 96 | 98 | 92 | |
| std. dev. | 3.2 | 4.1 | 4.4 | |
| overall average | 95.3 | |||
|
| ||||
2.4. Retention efficiency
- 2.4.1. Six glass fiber filters were spiked with 60 uL of a
6.26mg/mL solution of Parowax in carbon disulfide. This was
equivalent to a loading of 372 ug of paraffin wax which is
approximately the target concentration. They were stored overnight.
Eighty, 100 and 125 liters of humid air (70%) were drawn through the
filters the next day. The filters were extracted with carbon
disulfide and analyzed. The average result including all air volumes
is 101%. (Table 2)
Retention Study
|
| |||
| Sample | Air | Amount | % Retained |
| Number | Volume | Spiked | |
|
| |||
| 1 | 80 | 372 | 99 |
| 2 | " | " | 109 |
| 3 | 100 | " | 97 |
| 4 | " | " | 97 |
| 5 | 125 | " | 97 |
| 6 | " | " | 106 |
| average | 101 | ||
|
| |||
2.5. Storage
- 2.5.1. Five glass fiber filters were spiked with 60 uL of a 6.26
mg/mL solution of Parowax in carbon disulfide. This was equivalent
to a loading of 372 ug of paraffin wax which is approximately the
target concentration. They were stored in the refrigerator for five
days, extracted with carbon disulfide and analyzed. The average
recovery after five day storage is 95%. (Table 3)
Storage Study
|
| |||
| Sample | Amount | Days | % Recovered |
| Number | Spiked | Stored | |
|
| |||
| 1 | 372 | 5 | 94 |
| 2 | " | " | 91 |
| 3 | " | " | 101 |
| 4 | " | " | 95 |
| 5 | " | " | 93 |
| average | 95 | ||
|
| |||
2.6. Air volume and sampling rate studied
- 2.6.1. The air volume studied is 100 liters.
2.6.2. The sampling rate studied is 1 liter per minute.
2.7. Interferences
Suspected interferences should be listed on sample data sheets.
2.8. Safety precautions
- 2.8.1. Sampling equipment should be placed on an employee in a
manner that does not interfere with work performance or safety.
2.8.2. Safety glasses should be worn at all times.
2.8.3. Follow all safety practices that apply to the workplace being sampled.
3. Analytical method
- 3.1. Apparatus
- 3.1.1. Gas chromatograph equipped with a flame ionization
detector (FID).
3.1.2. GC column capable of separating the analyte and an internal standard, if used, from any interferences. The column used in this study was a DB-1 fused silica capillary having the following dimensions; 6 meter length, 0.32 mm ID, 1 u film.
3.1.3. An electronic integrator or some other suitable method of measuring peak areas.
3.1.4. Two milliliter vials with Teflon lined caps.
3.1.5. A 10 uL syringe or other convenient size for sample injection.
3.1.6. Pipettes for dispensing the desorbing solution. The Glenco 1 mL dispenser was used in this method.
3.1.7. Volumetric flasks - 10 mL and other convenient sizes for preparing standards.
3.2. Reagents
- 3.2.1. Purified GC grade nitrogen, hydrogen, and air.
3.2.2. Carbon disulfide, Reagent grade.
3.3.3. Parowax paraffin used as the analytical standard.
3.3. Sample preparation
- 3.3.1. Sample cassettes are opened and the filter is placed in a
4 mL vial.
3.3.2. Each filter is extracted with 2 mL carbon disulfide.
3.3.3. The vials are sealed immediately and extracted for 30 minutes with occasional shaking.
3.4. Standard preparation
- 3.4.1. Standards are prepared by diluting a known quantity of
Parowax in carbon disulfide. One thousand ug of Parowax in 10 mL of
carbon disulfide will give a standard equivalent to the TLV assuming
100 liter air volume and 2 mL extraction.
3.4.2. A range of separate standards should be made so that the sample results are bracketed.
3.5. Analysis
- 3.5.1. Gas chromatograph conditions.
| Flow rates (mL/min) | Temperature (deg.C) | ||
| Hydrogen carrier: | 1 | Injector: | 250 |
| Hydrogen flame: | 50 | Detector: | 300 |
| Air: | 300 | Column: | 250 |
| Nitrogen makeup: | 25 |
| Injection size: | 4 uL |
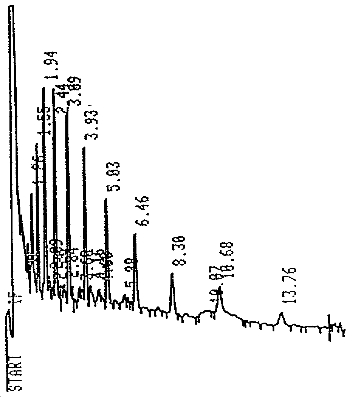
| Elution time: | Fingerprint from 2 to 12 min. |
| Chromatogram: | Figure 2 |
| Attenuation: | 2 |
3.5.2. Peak areas are measured by an integrator or other suitable means.
3.5.3. Precision was measured by by making 6 consecutive injections of 4 different standards containing Parowax concentrations of 37, 200, 372, and 751 ug/mL. The pooled coefficient of variation is 0.016. (Table 4)
Precision
|
| ||||
| Injection | 2.0x | 1.0x | 0.5x | 0.1x |
| Number | 750 ug/ml | 372 ug/ml | 200 ug/ml | 37 ug/ml |
|
| ||||
| 1 | 3852800 | 1939300 | 1056200 | 224100 |
| 2 | 3831200 | 1866400 | 1082500 | 219920 |
| 3 | 4044400 | 1902900 | 1059100 | 221010 |
| 4 | 4065500 | 1906900 | 1065500 | 227370 |
| 5 | 3686100 | 1074300 | 218380 | |
| 6 | 3694700 | 1071900 | 205610 | |
| Average = | 3862450 | 1903875 | 1068250 | 219398 |
| Standard | ||||
| Deviation = | 30425 | 25855 | 8963 | 6952 |
| CV = | .0079 | .014 | .0084 | .032 |
| Pooled CV = | .016 | |||
|
| ||||

A(1), A(2),A(3),A(4) = # of injections at each
level
CVl, CV2, CV3, CV4 = Coefficients at each level
3.6. Interferences (analytical)
- 3.6.1. Any compound having the general retention time of the
analyte or the internal standard used is an interference. Possible
interferences should be listed on the sample data sheet. GC
parameters should be adjusted if necessary so these interferences
will pose no problems.
3.6.2. Retention time data on a single column is not considered proof of chemical identity. Samples over the target concentration should be confirmed by GC/Mass Spec or other suitable means.
3.7. Calculations
- 3.7.1. To calculate the mg/m3 of analyte in samples,
the following equation should be used:
| (µg)
(L)(2 mL)(EE) |
= | mg
m3 |
| µg 2 mL 100 L EE |
= micrograms = Desorption volume = 100 liter air sample = Extraction efficiency |
3.8. Safety precautions
- 3.8.1. All handling of solvents should be done in a hood.
3.8.2. Avoid skin contact with all solvents.
3.8.3. Wear safety glasses at all times.
4. Recommendations for further study
The method seems adequate for both collection and analysis. The only thing that is left to look at is actual quantitative collection of fumes. Paraffin fumes have been produced by heating Parowax in a bubbler tube and collecting them on a filter attached to the exit port. The collection appeared to be good with no breakthrough to the backup pad. But no quantitative check has been performed using an actual dynamic atmosphere of paraffin fumes.
Much of the analytical work could possibly be avoided by the use of
pre- and post-weighings. It is unusual to have other contaminates in
areas where paraffin wax fumes are being emitted. By using weighings to
determine the total mass present on the filter it may be possible to
eliminate the chromatographic determination of samples with little or no
contamination. Most samples received at the lababoratory fall into this
category. This is an area that may warrant further study.
5. References
1) P. Shubik et al; "Studies on the Toxicity of Petroleum Waxes"; Toxicology and Applied Pharmacology; Aug. 2 1962.
2) "Encyclopedia of Chemical Toxicology", vol #24, Third Edition, Kirk-Othmer.
3) R. Prosser White "The Dermatergoses or Occupational Affections of the Skin" London H.K. Lewis & Co. 1934.
4) "Queries and Minor Notes" Journal of American Medical Assoc.
110:2102 1938.