THIOUREA
| Method number: | PV2059 |
| Matrix: | Air |
| Target Concentration: | 60 µg/m3 (arbitrary). There is no OSHA permissible exposure level (PEL) or ACGIH threshold limit value (TLV) for thiourea. |
| Procedure: | Samples are collected by drawing known volumes of air through a two-piece sampling cassette containing a glass fiber filter (GFF). The samples are extracted with methanol and analyzed by high performance liquid chromatograph (HPLC) using a ultraviolet (UV) detector. |
| Recommended air volume and sampling rate: |
480 L at 2.0 L/min |
| Detection limit of the overall procedure (based on the recommended air volume and the analytical detection limit): |
3.0 µg/m3 |
| Special precautions: | Samples should be shipped overnight and kept cold until analysis. |
| Status of method: | Partially validated method. This method is presented for information and trial use only. |
| Date: October 1993 (final) | Chemist: Ing-Fong Chan |
Organic Service Branch II
OSHA Salt Lake Technical
Center
Salt Lake City, UT 84165-0200
1. General Discussion
- 1.1. Background
- 1.1.1. History of procedure
In OSHA methods #95,"ETHYLENE THIOUREA" is collected on glass fiber filter (GFF) (Ref. 5. 1.). Because thiourea and ethylene thiourea are chemically similar, GFF was evaluated as a sampling medium for thiourea.
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
Chronic administration of thiourea in rats has resulted in hepatic tumors, bone marrow depression and goiters. Thiourea may reasonably be anticipated to be a carcinogen (Ref. 5.2.).
1.1.3. Potential workplace exposure
Thiourea is used as a photographic fixing agent and to remove stains from negatives; an intermediate in the manufacture of fire-retardant resins for lacy fabrics; vulcanization accelerator, and a reagent for determination of bismuth and selenite ions (Ref. 5.2. and 5.3.). There was no information available on the number of workers potentially exposed to thiourea.
1.1.4. Physical properties (Ref. 5.2. and 5.3.)
| Chemical name: | Thiocarbamide |
| Common name: | Thiourea |
| CAS number: | 62-56-6 |
| Molecular formula: | CH4N2S |
| Structural formula: | 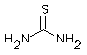 |
| Molecular weight: | 76.12 |
| Melting point: | 176-178°C |
| Solubility: | soluble in 11 parts water, in alcohol; sparingly soluble in ether. |
| Appearance: | colorless crystals |
1.2. Limit defining parameters
- 1.2.1. The detection limit of the analytical procedure
The detection limit of the analytical procedure is 4.9 ng per injection. This is the amount of analyte which gives a peak whose height is approximately five times the baseline noise.
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 1.47 µg per sample. This is the amount of analyte spiked on a glass fiber filter that, upon analysis, produces a peak similar in size to that of the detection limit of the analytical procedure.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. Samples are collected using a personal sampling pump that
can be calibrated within ±5% of the recommended flow rate with the
sampling device attached.
2.1.2. Samples are collected with a two-piece polystyrene cassette containing one glass fiber filter. These cassettes are commercially available from Millipore Coorporation.
2.2. Reagents
No sampling reagents are required.
2.3. Sampling technique
- 2.3.1. Prepare the sampler by removing the top and the end plugs
from the top and bottom pieces. Attach the sampler to the sampling
pump with a piece of flexible tubing and place it in the worker's
breathing zone.
2.3.2. After sampling for the appropriate time, remove the tube and reseal the cassette with the plastic plugs. Wrap each sample end-to-end with an OSHA seal (Form 21).
2.3.3. Record the air volume for each sample and list any possible interferences.
2.3.4. Submit at least one blank for each set of samples. The blank is handled in the same manner as the samples except no air is drawn through it.
2.3.5. Submit bulk samples for analysis in a separate container from the air samples.
2.4. Extraction efficiency
Three groups of four GFFs were each liquid spiked with 5 µL, 10 µL, and 20 µL of a 2.94 µg/µL solution of thiourea. These amounts represent 0.5, 1.0, and 2.0 times the target concentration respectively. They were transfered to 4-mL vials, sealed with polyethylene tetrafluoroethylene (PTFE)-lined caps and allowed to equilibrate overnight in a drawer at room temperature. The next day the samples were analyzed as per Section 3. The results are listed in Tables 2.4.1., 2.4.2., and 2.4.3. The average extraction efficiencies at 0.5, 1.0 and 2.0 times the target concentration are 0.918, 0.919, and 0.952 respectively.
Extraction Efficiency of Thiourea
at 0.5× Target Concentration
|
| |||
| sample i.d. |
µg spiked |
µg recovered |
recovery (decimal) |
|
| |||
| D1 D2 D3 D4 |
14.70 14.70 14.70 14.70 |
13.23 13.48 13.55 13.69 |
0.900 0.917 0.922 0.931 |
|
| |||
Extraction Efficiency of Thiourea
at 1× Target Concentration
|
| |||
| sample i.d. |
µg spiked |
µg recovered |
recovery (decimal) |
|
| |||
| D5 D6 D7 D8 |
29.40 29.40 29.40 29.40 |
26.77 26.96 27.03 27.29 |
0.910 0.917 0.919 0.928 |
|
| |||
Extraction Efficiency of Thiourea
at 2× Target Concentration
|
| |||
| sample i.d. |
µg spiked |
µg recovered |
recovery (decimal) |
|
| |||
| D9 D10 D11 D12 |
58.80 58.80 58.80 58.80 |
56.11 55.75 56.41 55.71 |
0.954 0.948 0.959 0.947 |
|
| |||
2.5. Retention efficiency
To test the ability of the sampler to retain the analyte, five
samplers were each liquid spiked with 10 µL of a 2.94
µg/µL solution of thiourea. All cassettes were sealed
with end-plug and allowed to equilibrate overnight in a drawer at room
temperature. The next day 480 L of humid air (![]() 80% relative
humidity) were drawn through each of the samples at 2.0 L/min. The
samples were analyzed as per section 3. The results are listed in
Table 2.5. The average retention efficiency from the five samplers was
0.937.
80% relative
humidity) were drawn through each of the samples at 2.0 L/min. The
samples were analyzed as per section 3. The results are listed in
Table 2.5. The average retention efficiency from the five samplers was
0.937.
Retention Efficiency of Thiourea
at 1× Target Concentration
|
| |||
| sample i.d. |
µg spiked |
µg recovered |
recovery (decimal) |
|
| |||
| RE-1 RE-2 RE-3 RE-4 RE-5 |
29.40 29.40 29.40 29.40 29.40 |
28.03 26.91 27.58 27.69 27.55 |
0.953 0.915 0.938 0.942 0.937 |
|
| |||
2.6. Sample storage
The storage samples were generated by liquid spiking each of ten
GFF with 10 µL of a 2.94 µg/µL solution of
thiourea. All cassettes were sealed with end-plugs and allowed to
equilibrate overnight in a drawer at room temperature. The next day
480 L of humid air ![]() 80%
80%
Refrigerator Storage
|
| |||
| days of storage |
µg spiked |
µg recovered |
recovery (decimal) |
|
| |||
| 8 8 8 8 8 |
29.40 29.40 29.40 29.40 29.40 |
28.34 27.25 27.06 27.12 27.12 |
0.964 0.927 0.921 0.922 0.922 |
|
| |||
Ambient Storage
|
| |||
| days of storage |
µg spiked |
µg recovered |
recovery (decimal) |
|
| |||
| 8 8 8 8 8 |
29.40 29.40 29.40 29.40 29.40 |
22.65 25.43 24.42 24.29 24.58 |
0.770 0.865 0.831 0.826 0.836 |
|
| |||
2.7. Recommended air volume and sampling rate
- 2.7.1. The recommended air volume is 480 L.
2.7.2. The recommended flow rate is 2.0 L/min.
2.8. Interferences (sampling)
It is not known if any compounds will interfere with the collection of thiourea. Any suspected interferences should be reported to the laboratory.
2.9. Safety precautions (sampling)
- 2.9.1. Attach the sampling equipment in such a manner that it
will not interfere with work performance or employee safety.
2.9.2. Follow all safety practices that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A balance capable of weighing to the nearest hundredth of
a milligram. A Mettler AE240 balance was used in this evaluation to
prepare the concentrated standards.
3.1.2. Volumetric flasks, pipets, and syringes of various convenient sizes for preparing standards, making dilutions and making injections.
3.1.3. Glass vials, 4-mL with PTFE-lined caps.
3.1.4. An HPLC equipped with a variable UV detector, and a WISP auto-sampler were used in this evaluation.
3.1.5. An HPLC column capable of separating thiourea from any interferences. A ZORBAX CN column (4.6 × 250 mm) was used in this evaluation.
3.1.6. An electronic integrator, or some other suitable means for measuring detector response. The Waters 860 Data System was used in this evaluation.
3.2. Reagents
- 3.2.1. Methanol, HPLC grade. The solvent used in this evaluation
was Optima® grade, obtained from Fisher Scientific, Inc.
3.2.2. Isooctane, HPLC grade. The solvent used in this evaluation was Optima® grade, obtained from Fisher Scientific, Inc.
3.2.3. Isopropanol, HPLC grade. The solvent used in this evaluation was Optima® grade, obtained from Fisher Scientific, Inc.
3.2.4. Thiourea. Thiourea was obtained from Mallinckrodt, INC. with a purity of 100%.
3.3. Standard preparation
- 3.3.1. Prepare stock standards by weighing 10-20 mg of thiourea
in 10-mL volumetric flasks and diluting to volume with methanol.
3.3.2. Prepare analytical standards by diluting the stock standards with methanol. A 9.6 µg/mL standard solution corresponds to the target concentration.
3.3.3. Prepare a sufficient number of analytical standards to generate a calibration curve. Analytical standard concentrations must bracket sample concentrations.
3.4. Sample preparation
- 3.4.1. Transfer GFF to 4-mL vial.
3.4.2. Add 3.0 mL of methanol to each vial.
3.4.3. Cap the vials and shake them on a mechanical shaker for 30 min.
3.5. Analysis
- 3.5.1. Instrument conditions
| column: | ZORBAX CN |
| eluent: | isooctane/isopropanol/methanol 45:10:45 (v/v/v) |
| flow rate: | 1.0 mL/min |
| injection volume: | 10 µL |
| retention time: | 4.0 min |
| UV detector: | 234 nm |
| Chromatogram: | 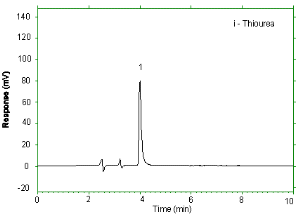 Chromatogram at the target concentration. |
3.6. Interferences (analytical)
- 3.6.1. Any compound that absorbs at 234 nm and has a similar
retention time as the analyte is a potential interference.
Generally, chromatographic conditions can be altered to separate an
interference from the analyte.
3.6.2. Retention time on a single column is not considered proof of chemical identity. Additional means of identification include: using an alternate HPLC column, detection at another wavelength (peak ratioing). Other possible means of identification might include gas chromatography/flame ionization detector or gas chromatography/mass spectrometry.
3.7. Calculations
- 3.7.1. Construct a calibration curve by plotting detector
response versus concentration (µg/mL) of thiourea.
3.7.2. Determine the µg/mL of thiourea in samples and blank from the calibration curve.
3.7.3. Blank correct each sample by subtracting the µg/mL found in the blank from the µg/mL found samples.
3.7.4. Determine the air concentration by using the following formulae.
| mg/m3 = | (µg/mL in sample) ×
(extraction volume, mL)
(air volume, L) × (extraction efficiency, decimal) |
| ppm = | (mg/m3)(24.46)
76.12 |
| where | 24.46 | = | molar volume (liters) at 101.3 kPa (760 mmHg) and 25°C |
| 76.12 | = | molecular weight of thiourea |
3.8. Safety precautions (analytical)
- 3.8.1. Avoid skin contact and air exposure to thiourea.
3.8.2. Avoid skin contact and air exposure with all solvents.
3.8.3. Wear safety glasses at all times in the laboratory.
4. Recommendation for Further Study
This method needs to be fully validated.
5. References
- 5.1. Chan, Y.; Methods #95, "ETHYLENE THIOUREA"; OSHA Analytical
Laboratory, unpublished, 1992
5.2. Budavari, S., O'Nell M.J., Smith, A., and Heckelman, P.E., The Merck Index, 11th ed., Merk & Co., Inc., Rahway, N.J., 1989; p 1476.
5.3. Bern, H.A., Boyland, E., and Brown, T.B. etc., IRAC Monographs: Evaluation Of Carcinogenic Risk, 1974; Vol. 7, pp. 95-105.