SULFUR DIOXIDE IN WORKPLACE ATMOSPHERES (BUBBLER)
| Method Number: | ID-104 |
| Matrix: | Air |
| OSHA Permissible Exposure Limits Sulfur Dioxide (Final Rule Limit): |
2 ppm (Time Weighted Average) 5 ppm (Short-Term Exposure Limit) |
| Sulfur Dioxide (Transitional Limit): | 5 ppm (Time Weighted Average) |
| Collection Device: | A calibrated personal sampling pump is used to draw a known
volume of air through a |
| Recommended Air Volume: | 15 to 60 L |
| Recommended Sampling Rate: | 1 L/min |
| Analytical Procedure: | Samples are directly analyzed with no sample preparation by ion chromatography as total sulfate. |
| Detection Limits Qualitative: |
0.0041 ppm (60-L air volume) |
| Quantitative: | 0.010 ppm (60-L air volume) |
| Precision and Accuracy Validation Level: |
2.5 to 10.0 ppm (60-L air volume) |
| CVT: | 0.012 |
| Bias: | -0.046 |
| Overall Error: | ±7% |
| Classification: | Validated Method |
| Chemists: | Ted Wilczek, Edward Zimowski |
| Date (Date Revised): | 1981 (December, 1989) |
Commercial manufacturers and products mentioned in this method are for descriptive use only and do not constitute endorsements byUSDOL-OSHA.
Similar products from other sources can be substituted.
OSHA Technical Center
Salt Lake City, Utah
1. Introduction
This method describes the collection and analysis of airborne sulfur
dioxide (SO2) using
- 1.1. History
An earlier method used by OSHA involved collecting
SO2 in 0.3 N hydrogen peroxide
(H2O2) which
converted SO2 to sulfuric acid. The amount
of SO2 in the air is determined in the
laboratory by volumetric titration of the sulfuric acid with barium
perchlorate and a Thorin indicator (8.1.). The titration is
susceptible to interferences from volatile phosphates and metals
(8.1.), and the end point is difficult to determine. Also, a report
indicated the chloride ion has an adverse effect on the endpoint
(8.2.). Method no.
1.2. Principle
Sulfur dioxide is collected in a MFGB containing 0.3 N H2O2. The H2O2 converts the SO2 to sulfuric acid (H2SO4) according to the following equation:
SO2 + H2O2 ---------> H2SO4
The H2SO4 is analyzed as sulfate using a slightly basic eluent and an ion chromatograph equipped with a conductivity detector.
1.3. Advantages and Disadvantages
- 1.3.1. This method has adequate sensitivity for measuring
workplace atmosphere concentrations of SO2
and is less affected by interferences found in the barium
perchlorate titration method.
1.3.2. The method can be fully automated to improve analytical precision.
1.3.3. Collected samples are analyzed by means of a quick instrumental method, since no sample preparation is required.
1.3.4. Humidity does not affect the collection efficiency.
1.3.5. The sulfuric acid formed is stable and non-volatile.
1.3.6. A disadvantage is the sampling device. The use of bubbler collection techniques may impose inconveniences for industrial hygiene work. There is the possibility of spillage during sampling, handling, and during transportation to the lab.
1.4. Potential sources of occupational exposure to SO2 (8.4., 8.5.) Sulfur dioxide is used in industry as a(n):
- intermediate in the manufacture of sulfuric acid
bleaching agent
disinfectant
fumigant
solvent
refrigerant
food preservative
reagent in the manufacture of magnesium, sodium sulfite, and other chemicals.
Sulfur dioxide is also an industrial by-product and can be generated from many industrial processes. These include the smelting of sulfide ores, the combustion of coal or fuel oils containing sulfur as an impurity, paper manufacturing, and petroleum refining (8.4.).
1.5. Physical Properties: Sulfur dioxide (CAS No. 7446-09-5) is a colorless, nonflammable gas with a characteristic, strong and suffocating odor. It is intensely irritating to the eyes and respiratory tract. It is soluble in water, methane, ethanol, chloroform, ethyl ether, acetic acid, and sulfuric acid (8.4., 8.5.).
| Chemical Formula: | SO2 | |
| Formula Weight: | 64.07 | |
| Boiling Point: | -10.0 °C | |
| Melting Point: | -72.7 °C | |
| Vapor Density: | 2.3 (air = 1) |
2. Range and Detection Limit (8.6.)
This method was evaluated over the range of 2.5 to 10.0 ppm
(atmospheric conditions of 640 mmHg and 24 °C). Total air sample volumes
of 60 L were used. The analytical portion of the evaluation was conducted
using a model 10 ion chromatograph with a 3 ×
- 2.1. The sensitivity of the method for the instrumentation used
during the validation study was 1.5 microsiemens/cm/µg as sulfate ion.
A 100 µL injection of a 10 µg/mL solution of sulfate gave a
2.2. The qualitative detection limit of the analytical method was
0.013 µg of SO2 per injection
2.3. The quantitative limit was 0.033 µg
SO2 per
3. Method Performance (8.6.)
This method was evaluated in 1981 using commercial analytical equipment mentioned in Section 2. Advances in ion chromatographic and sampling instruments should enable users to obtain similar or better results than those mentioned below.
- 3.1. The coefficient of variation (CVT)
for the overall sampling and analytical method in the range of 2.5 to
10 ppm (640 mmHg and 24 °C) was 0.012.
3.2. In validation experiments, this method was capable of measuring within ±25% of the true value (95% confidence level) over the validation range. The bias was -0.046 and overall error was ±7%.
3.3. The collection efficiency was 100% for the 0.3 N H202 sampling solution.
3.4. A breakthrough test was conducted at a concentration of 9.4 ppm. No breakthrough occurred after 240 min at a sampling rate of 1 L/min.
3.5. In storage stability studies, the average recovery of samples analyzed after 31 days were within 1% of the average recovery of samples analyzed immediately after collection.
4. Interferences
- 4.1. The presence of other particulate sulfate compounds and
sulfuric acid in the air will interfere in the analysis of sulfur
dioxide. These two interferences can be removed by the use of a
modified prefilter.
4.2. Sulfur trioxide gas (SO3), if present in a dry atmosphere, can give a positive bias in the SO2 determination.
4.3. Any substance that has the same retention time as the sulfate ion with the ion chromatographic operating conditions as described in this method is an interference. If the possibility of an interference exists, changing the separation conditions (column length, eluent flow rate and strength, etc.) may circumvent the problem.
4.4. When other substances are known or suspected to be present in the air sampled, the identities of the substances should be transmitted with the sample.
5. Sampling
- Sampling cassettes, polystyrene, 37-mm.
- Mixed-cellulose ester (MCE) filters, 37 mm.
- Support rings, cellulose, part no. 225-23 (SKC Inc., Eighty
Four, PA). Rings can also be made from 37-mm cellulose backup pads
- Place a
half-dollar in the center of the pad and then cut the outer ring formed. Place this ring in the cassette to provide support for the MCE filter.
- 5.1. Equipment
- 5.1.1. Hydrogen peroxide (30%
H2O2), reagent
grade or better.
5.1.2. Collection solution, 0.3 N H2O2. Carefully dilute 17 mL of 30% H2O2 solution to 1 L with deionized water.
5.1.3. Personal sampling pumps capable of sampling within ±5% of the recommended flow rate of 1 L/min are used.
5.1.4. Midget-fritted glass bubblers (MFGBs), 25-mL, part no. 7532 (Ace Glass Co., Vineland, NJ).
5.1.5. Shipping vials: Scintillation vials, 20-mL, part no. 74515 or 58515, (Kimble, Div. of Owens-Illinois Inc., Toledo, OH) with polypropylene or Teflon cap liners. Tin or other metal cap liners should not be used.
5.1.6. A stopwatch and bubble tube or meter are used to calibrate pumps.
5.1.7. Various lengths of polyvinyl chloride (PVC) tubing are used to connect bubblers to the pumps.
5.1.8. If particulate sulfate or sulfuric acid is suspected to also be in the atmosphere, a modified prefilter assembly is used. This assembly consists of:
5.2. Sampling procedure
- 5.2.1. Calibrate the sampling pump with a MFGB containing about
10 to 15 mL of collection solution
5.2.2. Place 10 to 15 mL of collection solution in an MFGB. Connect the MFGB to a calibrated sampling pump and then place the sampling device in the breathing zone of the employee.
5.2.3. If particulate sulfate or sulfuric acid are suspected to be present, attach the modified prefilter (Section 5.1.8.) to the MFGB with PVC tubing so that sampled air enters the cassette first. Minimize the amount of tubing from the filter to the MFGB.
5.2.4. Sample at a flow rate of 1 L/min. For STEL determinations, sample for at least 15 min. For measurements of TWA exposures, sample from 60 to 240 min. Take enough samples to cover the shift worked by the employee.
5.2.5. Transfer the collection solution into a 20-mL glass
scintillation vial. Rinse the bubbler with 2 to 3 mL of unused
collection solution and transfer the rinsings into the sample vial.
Place the Teflon- or
5.2.6. Prepare blank solutions by taking 10 to 15 mL of the
unused collection solution and transfer to individual
5.2.7. Request sulfur dioxide analysis on the OSHA 91A form. If sulfuric acid is also suspected in the sampled atmosphere and a prefilter assembly was used, the MCE filter can be submitted for sulfuric acid analysis.
5.2.8. Ship the samples to the laboratory using appropriate packing materials to prevent breakage.
6. Analysis
- 6.1. Precautions
- 6.1.1. Refer to instrument and standard operating procedure
(SOP) manuals (8.7.) for proper operation.
6.1.2. Observe laboratory safety regulations and practices.
6.1.3. Sulfuric acid (H2SO4) can cause severe burns. Wear protective eyewear, gloves, and labcoat when using concentrated H2SO4.
6.2. Equipment
- 6.2.1. Ion chromatograph (model no. 2010i or 4500, Dionex,
Sunnyvale, CA) equipped with a conductivity detector.
6.2.2. Automatic sampler (model no. AS-1, Dionex) and 0.5 mL sample vials (part no. 038011, Dionex).
6.2.3. Laboratory automation system: Ion chromatograph interfaced to a data reduction and control system (model no. AutoIon 450, Dionex).
6.2.4. Micromembrane suppressor (model no. AMMS-1, Dionex).
6.2.5. Anion separator column (model no. HPIC-AS4A, Dionex) with
pre-column (model no.
6.2.6. Disposable syringes (1 mL) and syringe pre-filters, 0.5 µm pore size, (part no. SLSR 025 NS, Millipore Corp., Bedford, MA).
| (Note: | Some syringe pre-filters are not cation- or anion-free. Tests should be done with blank solutions first to determine suitability for the analyte being determined). |
6.2.7. Miscellaneous volumetric glassware: Micropipettes, volumetric flasks, graduated cylinders, and beakers.
6.2.8. Analytical balance (0.01 mg).
6.3. Reagents - All chemicals should be at least reagent grade.
- 6.3.1. Deionized water (DI H2O) with a
specific conductance of less than 10 microsiemens.
6.3.2. Eluent [0.0015 M sodium carbonate (Na2CO3)/0.0015 M sodium bicarbonate (NaHCO3)]: Dissolve 0.636 g Na2CO3 and 0.504 g NaHCO3 in 4.0 liters of DI H2O.
6.3.3. Sulfuric acid (H2SO4), concentrated (98%).
6.3.4. Regeneration solution (0.02 N
H2SO4): Pipet
1.14 mL concentrated
H2SO4 into a
6.3.5. Sodium sulfate (Na2SO4).
6.3.6. Sulfate stock standard (1,000 µg/mL sulfate): Dissolve and
dilute 1.4792 g
Na2SO4 to
6.4. Standard Preparation
Working standards (100, 10, 1.0, and 0.1 µg/mL as sulfate). Make appropriate serial dilutions of the sulfate stock standard with eluent. Prepare these solutions monthly.
6.5. Sample Preparation
- 6.5.1. Measure and record the total solution volume of each
sample with a graduated cylinder.
6.5.2. If the sample solutions contain suspended particulate,
remove the particles using a
6.5.3. Fill the 0.5-mL automatic sampler vials with sample solutions and push a filtercap into each vial. Label the vials.
6.5.4. Load the automatic sampler with labeled samples, standards and blanks.
6.6. Analysis
Set up the ion chromatograph and analyze the samples and standards in accordance with the SOP (8.7.). Typical operating conditions for a Dionex 2010i with a data reduction system are listed below.
| Ion chromatograph | |
| Eluent: | 0.0015 M Na2CO3/0.0015 M NaHCO3 |
| Column temperature: | ambient |
| Conductivity detector Sensitivity: |
1 to 3 microsiemens |
| Micromembrane Suppressor | |
| Regenerant flow: | 3 to 5 mL/min |
| Gas pressure: | 5 to 10 psi |
| Pump | |
| Pump pressure: | approximately 1,000 psi |
| Flow rate: | 2 mL/min |
| Chromatogram | |
| Run time: | 6 min |
| Sample injection loop: | 50 µL |
| Average retention time Sulfate: |
approximately 5.4 min |
Analyze a standard in the concentration range of the samples after every fourth or fifth sample and at the end of the analysis.
7. Calculations
- 7.1. Hard copies of chromatograms containing peak area and height
data should be obtained from a printer. A typical chromatogram is
shown in Figure 1.
7.2. Using a least squares regression program, prepare a concentration-response curve by plotting the concentration of the prepared µg/mL values of the standards (or µg/sample if the same injection and solution volumes are used for samples and standards) versus peak areas or peak heights. Calculate sample concentrations from the curve and blank correct all samples as shown:
C µg SO4 2 ¯ = (S µg/mL)(SSV) - (BL µg/mL)(BLSV)
| Where: | ||
| C µg SO4 2 ¯ | = | Corrected amount (µg) in the sample solution. |
| S µg/mL | = | µg/mL sample (from curve) |
| SSV | = | Sample solution volume (from Section 6.5.1.) |
| BL µg/mL | = | µg/mL blank (from curve) |
| BLSV | = | Blank solution volume (from Section 6.5.1.) |
7.3.The concentration of SO2 in each air sample is expressed in ppm and is calculated as:
| ppm SO2 = | MV × C µg
SO42¯ × Conversion
formula weight × air volume |
| Where: | ||
| MV (Molar Volume) | = | 24.45 (@ 25 °C and 760 mmHg) |
| C µg SO42 ¯ | = | blank corrected sample result |
| Gravimetric conversion (SO42 ¯ to SO2) |
= | 0.667 |
| Formula Weight (SO2) | = | 64.07 |
| Air Volume | = | Air sample taken (in L) |
This equation reduces to:
| ppm SO2 = | 0.2545 × C µg
SO42¯
air volume |
7.4.Reporting Results
Results are reported to the industrial hygienist as ppm sulfur dioxide.
8. References
- 8.1. National Institute for Occupational Safety and Health:
NIOSH Manual of Analytical Methods. 2nd. ed., Vol. 4 (Method
No. S308) (DHEW/NIOSH Pub. No.
8.2. Steiber, R. and R. Merrill: Application of Ion
Chromatography to the Analysis of Source Assessment Samples. In Ion
Chromatographic Analysis of Environmental Pollutants (Volume 2),
edited by J.D. Mulik & E. Sawicki. Ann Arbor, MI: Ann Arbor
Science Publishers Inc., 1979. pp.
8.3. Occupational Safety and Health Administration Analytical
Laboratory: OSHA Analytical Methods Manual
(USDOL/OSHA-SLCAL Method No.
8.4. National Institute for Occupational Safety and Health:
Criteria for a Recommended Standard -- Occupational Exposure to
Sulfur Dioxide (DHEW/NIOSH Pub. No.
8.5. Fassett, D.W. and D.D. Irish, ed.: Patty's Industrial Hygiene and Toxicology. 2nd rev. ed., Vol. 2. New York: John Wiley and Sons, 1963.
8.6. Occupational Safety and Health Administration Technical Center: Sulfur Dioxide Backup Data Report (ID-104). Salt Lake City, UT. Revised, 1989.
8.7. Occupational Safety and Health Administration Technical Center: Ion Chromatography Standard Operating Procedure. Salt Lake City, UT. In progress (unpublished).
| Chloride | 3 µg | |||||||
| Nitrate | 20 µg | |||||||
| Phosphate | 20 µg | |||||||
| Sulfate | 20 µg | |||||||
| REPORT | VOLUME | DILUTION | POINTS | RATE | START | STOP | AREA REJ | |
|
| ||||||||
| External | 1 | 1 | 1863 | 5Hz | 0.00 | 6.21 | 500000 | |
| Pk. Num |
Ret Time |
Component Name |
Height | Area | ||||
|
| ||||||||
| 1 2 3 4 5 6 |
0.27 0.85 1.35 2.47 3.98 5.37 |
chloride nitrate phosphate sulfate |
14570313 460000 1126875 21717447 6749818 15340000 |
90797038 2628000 8174000 213280000 92956000 258840000 | ||||
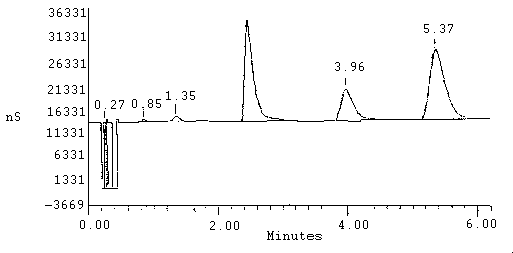
Figure 1