|
PARTICULATE MERCURY IN
WORKPLACE ATMOSPHERES
| Method Number: |
ID-145 |
| Matrix: |
Air*, Wipe, or Bulk |
OSHA Permissible Exposure
Limits
Aryl and Inorganic Compounds
of
Mercury (Final Rule Limit): |
0.1
mg/m3 as mercury
(Ceiling) |
|
Also Skin Designation |
Mercury
(Transitional
Limit): |
0.1
mg/m3 as mercury (Time
Weighted Average) |
| Sampling (Air Samples) |
|
| Collection Device: |
A
mercury-containing ester
filter and a calibrated personal sampling pump are
used. |
| Recommended Sampling
Rate: |
2.0 L/min |
| Recommended Air
Volume: |
10 L |
| Analytical Procedure: |
The collection medium is digested
using nitric and sulfuric acids. Potassium permanganate
and hydroxylamine hydrochloride are then added. The
mercury in the sample is reduced using stannous chloride
and analyzed using a cold
vapor-atomic absorption
spectrophotometer. |
| Detection Limit: |
|
| Qualitative: |
0.001 mg/m3
for a 10 L air sample |
| Quantitative: |
0.002 mg/m3
for a 10 L air sample |
| Precision and Accuracy (from
samples prepared with phenyl mercuric acetate) |
|
CV1 |
0.086 |
| Bias |
+0.033 |
| Method Classification: |
Validated Method |
| Date (Date Revised): |
1987 (December,
1989) |
| * |
If mercury vapor is also suspected to
be present, OSHA Method No. ID-140 should also be
consulted. |
Commercial manufacturers
and products mentioned in this method are for
descriptive
use only and do not constitute endorsements by
USDOL-OSHA.
Similar products from other sources can be
substituted.
Division of Physical
Measurements and Inorganic Analyses
OSHA Technical
Center
Salt Lake City, Utah
1. Introduction
This method
describes the collection of airborne particulate mercury on
0.8-µm mixed-cellulose ester membrane (MCE)
filters and the subsequent analysis using a cold
vapor-atomic absorption spectrophotometer
(CV-AAS). Wipe and bulk samples can also be collected and
analyzed for mercury content using this method.
1.1. History
This method is an
adaptation of an analytical technique proposed by Hatch
and Ott (8.1.). Particulate mercury contained in air
samples, as well as wipe and bulk samples have always been
analyzed at the OSHA Salt Lake Technical Center
(OSHA-SLTC) using CV-AAS. Elemental mercury vapor is
sampled and analyzed using techniques described in
reference 8.2.
1.2. Principle
Airborne and
non-airborne particulate mercury compounds and
mercury-containing dust are collected, respectively, on
0.8-µm MCE and wipe filters. Bulk material is collected by
grab sampling. Air filter, wipe, and bulk samples are
initially dissolved using concentrated nitric and sulfuric
acids. A potassium permanganate solution is added to help
dissolve the sample matrix. A hydroxylamine hydrochloride
solution is then added to reduce the excess potassium
permanganate. Finally, stannous chloride is added to an
aliquot of the sample to reduce the mercury to the vapor
state. This vapor is then driven into an absorption cell
of a flameless atomic absorption spectrophotometer for
analysis.
1.3. Advantages and Disadvantages
1.3.1. The air sampling device is small and
portable.
1.3.2. This method has adequate
sensitivity for measuring workplace exposure to
mercury-containing dust and particulate mercury
compounds.
1.3.3. Sample preparation for analysis
involves simple procedures.
1.3.4. Particulate
organo-mercury compounds may also be collected using 0.8
µm MCE filters.
1.3.5. Elemental mercury vapor
cannot be collected on the 0.8 µm MCE filters. If
elemental mercury vapor is suspected to be present, a
sample using HydrarR or
hopcalite solid sorbent as the collection medium should
be separately taken (8.2.).
1.4. Workplace
Exposure
Occupations with potential exposure to
mercury and its compounds are listed (8.3.):
|
amalgam
makers
bactericide makers
barometer
makers
battery makers, mercury
boiler
makers
bronzers
calibration instrument
makers
cap loaders , percussion
carbon brush
makers
caustic soda makers
ceramic
workers
chlorine makers
dental amalgam
makers
dentists
direct current meter
workers
disinfectant makers
disinfectors
drug
makers
dye makers
electric apparatus
makers
electroplates
embalmers
explosive
makers
farmers
fingerprint
detectors
fireworks makers
fungicide
makers
fur preservers |
fur processors
gold
extractors
histology technicians
ink
makers
insecticide makers
investment casting
workers
jewelers
laboratory workers,
chemical
lampmakers, fluorescent
manometer
makers
mercury workers
miners, mercury
neon
light makers
paint makers
paper
makers
percussion cap makers
pesticide
workers
photographers
pressure gage
makers
refiners, mercury
seed handlers
silver
extractors
switch makers, mercury
tannery
workers
taxidermists
textile
printers
thermometer makers
wood preservative
workers |
1.5. Toxic Effects
Note: Information listed within this
section is a synopsis of current knowledge of the
physiological effects of mercury and is not intended to be
used as a basis for OSHA policy.
Exposure to elemental mercury vapor can
occur via the respiratory tract and skin. Possible symptoms
from an acute exposure include severe nausea, vomiting,
abdominal pain, bloody diarrhea, kidney damage, and death.
These symptoms usually present themselves within 10 days of
exposure. Potential symptoms from a chronic exposure include
inflammation of the mouth and gums, excessive salivation,
loosening of the teeth, kidney damage, muscle tremors, jerky
gait, spasms of the extremities, personality changes,
depression, irritability, and nervousness (8.3.,
8.4.).
1.6. Properties (8.3.-8.5.)
Elemental
mercury (CAS No. 7439-97-6) is a silver-white, heavy, mobile,
liquid metal at room temperature. Some physical properties and
data for mercury are:
|
Atomic Number |
80 |
|
Atomic Symbol |
Hg |
|
Atomic Weight |
200.61 |
|
Freezing Point |
-38.87 °C |
|
Boiling Point |
356.90 °C |
|
Density |
13.546 g/mL (20 °C) |
|
Synonyms |
Quicksilver,
Hydrargyrum |
Many different inorganic and aryl
compounds of mercury exist. One of the more common aryl
compounds is phenyl mercuric acetate
(C6H5HgOCOCH3)
which is used as a fungicide:
|
CAS no. |
62-38-4 |
|
Atomic Weight |
336.75 |
|
Melting Point |
149 °C |
|
Volatility |
slightly volatile at room temp. |
|
Solubility |
soluble in alcohol, benzene,
and
glacial acetic acid, slightly
soluble in
water |
2. Range
2.1. Detection Limits
The
qualitative and quantitative detection limits for the
analytical procedure are 0.01 µg and 0.02 µg mercury,
respectively (8.6.).
2.2. Working Range
The
range of the analytical procedure has been determined to be
0.1 to 2 µg mercury. Using the analytical conditions
specified, a nonlinear response was noted above 2 µg.
3. Method
Performance
3.1. The average recovery of 88 quality
control samples containing mercury spiked on MCE filters in
the approximate range of 1 to 2 times the OSHA PEL (assuming
a 20-L air volume) was 90.8% with a coefficient of variation
(CV1) of 0.149 (8.7.). The
variability and slight decrease in recovery for these
samples, which were prepared from 1984 to 1986, was
attributed to instability during storage. These samples had
been spiked with dilute nitric acid solutions containing
mercury and some samples were analyzed months after
preparation.
3.2. An additional test to determine
method performance was conducted using spikes of phenyl
mercuric acetate (PMA) on 24 MCE filters (8.8.). The filters
were spiked with PMA solutions containing 50 to 200 µg total
mercury. The recovery for 18 of the samples was 103.3% and a
coefficient of variation of 0.086. The remaining six samples
were subjected to a retention efficiency test where air was
drawn through the spiked filters for 3 h. The filters were
then analyzed. Results indicated the filters sufficiently
retained the PMA . 4.
Interferences
Organic-free deionized water should
be used during sample and standard preparation. Any compound
with the same absorbance wavelength as mercury (253.7 nm) can
be a positive interference. Some volatile organic compounds
(i.e. benzene, toluene, acetone, carbon tetrachloride) absorb
at this wavelength and are considered analytical
interferences. They occur as contaminants in the reagents used
during sample preparation. These compounds are not expected to
be retained on an MCE filter during sample collection.
Analytical interferences are rendered insignificant by using
organic-free deionized water and at least reagent grade
chemicals or by blank subtraction.
Increasing the
concentration of nitric acid in the samples or standards
appears to produce an elevated background signal. The nitric
acid concentration in the samples and standards should not be
greater than 10%.
5. Sampling - Particulate
Mercury
For the sampling and analysis of mercury
vapor, consult reference 8.2.
5.1. Equipment - Air Filters
5.1.1. Filters: Mixed-cellulose ester
(MCE) filters (0.8-µm pore size), cellulose backup pads,
and cassettes, 37-mm diameter (part no. MAWP 037 A0,
Millipore Corp., Bedford, MA).
5.1.2. Gel bands
(Omega Specialty Instrument Co., Chelmsford, MA) for
sealing cassettes.
5.1.3. Sampling pumps capable of
sampling at 2 liters per minute (L/min).
5.1.4.
Assorted flexible tubing.
5.1.5. Stopwatch and
bubble tube or meter for pump calibration.
5.2.
Equipment - Wipe Samples
Smear tabs (part no. 225-24,
SKC Inc., Eighty Four, PA), or wipe filters (Whatman no. 41
or no. 42 filters, Whatman LabSales Inc., Hillsboro,
OR).
5.3. Equipment - Bulk
Samples
Scintillation vials, 20-mL (part no. 74515 or
58515, Kimble, Div. of Owens-Illinois Inc., Toledo, OH) with
polypropylene or Teflon cap liners. If possible, submit bulk
or wipe samples in these vials. Tin or other metal cap
liners should not be used since amalgamation can occur
between the metal and mercury.
5.4. Sampling
Procedure - Air Samples
5.4.1. Place a MCE filter and a cellulose backup pad
in each two- or three-piece cassette. Seal each cassette
with a gel band.
5.4.2. Calibrate each personal
sampling pump with a prepared cassette in-line to
approximately 2 L/min.
5.4.3. Attach prepared
cassettes to calibrated sampling pumps (the backup pad
should face the pump) and place in appropriate positions
on the employee or workplace area.
5.4.4. Collect
the samples for at least 5 min.
5.4.5. Place
plastic end caps on each cassette after sampling. Attach
an OSHA-21 seal around each cassette in such a way as to
secure the end caps.
5.5. Sampling Procedure -
Wipe Samples
A skin designation has been assigned to
these mercury-containing compounds.
5.5.1. Wear clean, impervious, disposable gloves when
taking each wipe sample.
5.5.2. Moisten the wipe
filters with deionized water prior to use.
5.5.3.
If possible, wipe a surface area covering 100
cm2.
5.5.4. Fold the wipe
sample with the exposed side in.
5.5.5. Transfer
the wipe sample into a 20-mL scintillation vial and seal
with vinyl or electrical tape. Securely wrap an OSHA-21
seal length-wise from vial top to bottom.
5.6.
Sampling Procedure - Bulk Samples
In order of
laboratory preference, bulk samples may be one of the
following:
1) a high-volume filter sample,
2) a
representative settled dust (rafter) sample,
3) a sample
of the bulk material in the workplace.
5.6.1. Transfer the bulk material into a 20-mL
scintillation vial and seal with vinyl or electrical tape.
Securely wrap an OSHA-21 seal length-wise from vial top to
bottom.
5.6.2. The type of bulk sample should be
stated on the OSHA 91 and cross-referenced to the
appropriate air sample(s).
5.7.
Shipment
5.7.1. Submit at least one blank sample with each set
of air or wipe samples. Blank filter samples should be
handled in the same manner as other samples, except that
an air or wipe sample is not taken.
5.7.2. Send the
samples to the laboratory as soon as possible with the
OSHA 91A paperwork requesting particulate mercury
analysis.
5.7.3. Bulk samples should be shipped
separately from air samples. They should be accompanied by
Material Safety Data Sheets if available. Check current
shipping restrictions and ship to the laboratory by the
appropriate method. 6.
Analysis
6.1. Safety Precautions
6.1.1. Wear safety glasses, labcoat, and gloves at all
times.
6.1.2. Handle acid solutions with care.
Avoid direct contact of acids with work area surfaces,
eyes, skin, and clothes. Flush acid solutions which
contact the skin or eyes with copious amounts of cold
water.
6.1.3. Prepare solutions containing
hydrochloric acid in an exhaust hood and store in
narrow-mouthed bottles.
6.1.4. Keep B.O.D. bottles
containing stannous chloride/hydrochloric acid solutions
capped when not in use to prevent inhalation of noxious
vapors.
6.1.5. Exercise care when using laboratory
glassware. Do not use chipped pipets, volumetric flasks,
beakers or any glassware with sharp edges
exposed.
6.1.6. Never pipet by mouth.
6.1.7.
Always purge the mercury from the CV-AAS into an exhaust
vent.
6.1.8. Occasionally monitor the CV-AAS for
mercury vapor leaks using an appropriate direct-reading
instrument.
6.2. Equipment - Cold Vapor
Analysis
(Note: Specific equipment is listed for
illustration only)
6.2.1. Atomic absorption spectrophotometer (model 503,
Perkin-Elmer, Norwalk, CT).
6.2.2. Mercury hollow
cathode lamp or electrodeless discharge lamp and power
supply.
6.2.3. Biological Oxygen Demand (B.O.D.)
bottles, borosilicate glass, 300 mL.
6.2.4.
Peristaltic pump, 1.6 to 200 mL range, and controller,
1-100 rpm range (Masterflex model 7553-30 with model 7015
head, Cole-Parmer, Chicago, IL).
6.2.5. Quartz
absorption cell, 22 mm (7/8 in) o.d. X 152 mm (6 in) long
(part no. 303-3101,
Perkin-Elmer).
6.2.6. Heating tape.
6.2.7.
Variable transformer 50-60 Hz, single phase, 10 A, 120 V
input, 0-140 V output, 1.4 kW (Superior Electric, Bristol,
CT).
6.2.8. Tygon peristaltic pump tubing (part no.
N06409-15, Cole-Parmer), and glass tubing.
6.2.9.
Aerator (part no. 0303-3102, Perkin-Elmer).
6.2.10.
Chart recorder.
6.2.11. Desiccant (Drierite, W.A.
Hammond Drierite Co., Xenia, OH).
6.2.12.
Volumetric flasks, volumetric pipets, beakers, and other
laboratory glassware.
6.2.13. Automatic pipets,
adjustable, 0.1 to 5.0 mL range (models P-1000 and P-5000,
Rainin Instruments Co., Woburn, MA).
6.2.14.
Exhaust vent.
6.2.15. Automatic pipets, glass or
Teflon, unlubricated (cat. no. 050-03-908-1, Brinkmann
Dispensette, Brinkmann Instruments, Westbury,
NY).
6.2.16. Phillips beakers, 250
mL.
6.2.17. Hot plate (used only for cleaning
glassware, not for sample digestions).
6.2.18.
Analytical balance (0.01 mg).
6.3. Reagents - All
reagents should be at least reagent grade.
Potassium
permanganate
(KMnO4)
Hydroxylamine
hydrochloride
(NH2OH.HCl)
Stannous
chloride (SnCl2)
6.3.1. Deionized water (DI
H2O),
organic-free.
6.3.2. Hydrochloric acid (HCl),
concentrated (36.5 to 38%), with a mercury concentration
less than 0.005 ppm.
6.3.3. Hydroxylamine
hydrochloride
(NH2OH.HCl)
solution, 20%: Dissolve 200 g
NH2OH.HCl in
DI H20 and dilute to 1 L. A
glass automatic pipet is useful in dispensing this
reagent. Rinse the automatic pipet dispenser with DI
H2O after the analysis is
completed.
6.3.4. Mercury standard stock solution,
1,000 µg/mL: Use a commercially available certified
standard or, alternatively, dissolve 1.0798 g of dry
mercuric oxide (HgO) in 50 mL of 1:1 hydrochloric acid and
then dilute to 1 Lwith DI
H2O. Store this reagent
in a dark environment, preferably in an amber colored
container.
6.3.5. Nitric acid
(HNO3), concentrated (69 to
71%), with a mercury concentration less than 0.005
ppm.
6.3.6. Nitric acid, 1:1: Carefully add equal
portions of concentrated HNO3
and DI
H2O.
6.3.7. Nitric
acid, 10%: Carefully add 100 mL of concentrated
HNO3 to 900 mL of DI
H2O.
6.3.8.
Potassium permanganate (KMnO4)
solution, 5%: Dissolve 50 g
KMnO4 in 1 L DI
H2O. This concentration
is near saturation and the crystals will dissolve slowly.
Stirring with a magnetic stirring bar/stirrer is
recommended. An unlubricated glass automatic pipet is
useful in dispensing the reagent during the analysis.
After the analysis is completed, rinse and clean the
automatic pipet dispenser to prevent seizing. Remove any
dark deposits produced by permanganate reduction products
with a 20% hydroxylamine hydrochloride
solution.
6.3.9. Stannous chloride
(SnCl2) solution, 10%: Dissolve
20 g SnCl2 in 100 mL
concentrated HCl. Slowly and carefully pour this solution
into 100 mL DI H2O
and then mix well. Transfer and store the final solution
in a capped B.O.D. bottle to prevent oxidation. Prepare
this solution before each new analysis.
6.3.10.
Sulfuric acid
(H2SO4),
concentrated (95 to 98%), with a mercury concentration
less than 0.005 ppm.
6.3.11. Sulfuric acid
(H2SO4),
5 M: Cautiously add 278 mL concentrated
H2SO4
to approximately 600 mL DI
H2O. Allow the solution
to cool to room temperature and then dilute slowly to 1
L.
6.4. Glassware Preparation
6.4.1. Clean the 250-mL Phillips beakers by refluxing
with 1:1 HNO3 on a hot plate in
an exhaust hood. Thoroughly rinse with DI
H2O and allow to
dry.
6.4.2. Clean the B.O.D. bottles and stoppers
with 1:1 HNO3 and thoroughly
rinse with DI H2O
prior to use.
6.4.3. Rinse all other glassware with
10% HNO3 and then with DI
H2O prior to use.
6.5.
Standard Preparation
6.5.1. Prepare a 1 µg/mL mercury standard by making
appropriate ten-fold serial dilutions of the 1,000 µg/mL
mercury standard stock solution with 10%
HNO3.
6.5.2. Prepare
working mercury standards (ranging from 0.1 to 2.0 µg) and
reagent blanks immediately prior to use. A few
standards at each concentration should be prepared. Add an
appropriate aliquot of the 1 µg/mL standard to a clean
B.O.D. bottle containing enough 10%
HNO3 to bring the total volume
to 100 mL. A suggested dilution scheme is given:
|
| Standard
(µg) |
Aliquot
(mL)* |
Final
Volume (mL) |
|
| Reagent Blank |
0 |
100 |
| 0.1 |
0.1 |
100 |
| 0.2 |
0.2 |
100 |
| 0.5 |
0.5 |
100 |
| 1.0 |
1.0 |
100 |
| 1.5 |
1.5 |
100 |
| 2.0 |
2.0 |
100 |
*
Aliquot taken from 1 µg/mL standard prepared in
Section 6.5.1. |
| 6.6.
Sample Preparation
Sample digestion is performed at
room temperature under oxidizing conditions to avoid loss of
mercury.
6.6.1. Perform the transfer and digestion
of samples in an exhaust hood . Transfer air and wipe
samples, and previously weighed aliquots of bulk samples to
separate labeled 250 mL Phillips beakers.
6.6.2. Add
5 mL conc. HNO3 to each Phillips
beaker and allow to stand for a few min. Subsequently add 15
mL of 5 M
H2SO4
followed by the appropriate amount of 5%
KMnO4 to each sample to completely
oxidize any additional organic material:
| Bulk samples |
45 mL of 5%
KMnO4 |
| Large (> 4 cm diameter) wipe
samples |
45 mL of 5%
KMnO4 |
| Air, small wipe, and smear tab
samples |
20 mL of 5%
KMnO4 | Swirl the samples frequently to break up the
collection media and allow the samples to digest at least 1 h
at room temperature.
6.6.3. Add the appropriate amount
of 20% NH2OH.HCl
to each Phillips beaker:
| Bulk samples |
10 mL |
| Large (> 4 cm diameter) wipe
samples |
10 mL |
| Air, small wipe , and smear tab
samples |
5 mL |
The solution and suspended matter in each
Phillips beaker should lose the dark-brown or purple color
resulting from the KMnO4 treatment
and become clear. If it does not, add
NH2OH.HCl crystals
directly to the beaker until clear.
6.6.4.
Quantitatively transfer the sample solution from each Phillips
beaker to a 100-mL volumetric flask and dilute to
volume. This is a good place to stop if the analysis cannot be
completed the same day.
6.7. Analysis - Instrument
Parameters
6.7.1. Set up the CV-AAS as illustrated in
Figure 1.
6.7.2. Wrap the heating tape around the
quartz cell and then turn on the variable transformer. The
heat setting on the tape should be sufficient to prevent
water vapor condensation in the absorption
cell.
6.7.3. Place the aerator in a B.O.D. bottle
which contains approximately ½ to 1 inch of desiccant.
Operate the peristaltic pump for approximately 30 min at
full speed to remove any water vapor from the
system.
6.7.4. Operate the hollow cathode or
electrodeless discharge mercury lamp at the manufacturer's
recommended current or power rating.
6.7.5. Use the
following settings (Note: The mentioned instrument settings
are for specific models used at the OSHA-SLTC. If
instrumentation other than what is specified in Section 6.2.
is used, please consult the instrument manufacturer's
recommendations.):
| Atomic Absorption
Spectrophotometer: |
| Slit |
0.7 nm |
| Signal |
Repeat Mode |
| Function |
ABS |
| Mode |
ABS |
| Range |
UV |
| Wavelength |
253.7 nm |
| Filter |
Out |
| EM Chopper |
Off |
| Phase |
Normal |
Strip Chart
Recorder: |
| Chart Speed |
5 mm/min |
| Chart Range |
10
mV |
6.7.6. Optimize the ENERGY meter reading at
253.7 nm.
6.7.7. Align the beam of the mercury lamp so
it passes directly through the center of the quartz cell
windows. This can be accomplished by adjusting the burner
height, depth, and angle knobs to give a minimum ABSORBANCE
reading.
6.7.8. Operate the peristaltic pump at full
speed. Rinse the aerator with DI
H2O and insert it into a
holder in the exhaust vent.
6.7.9. Perform the
following steps to obtain a baseline signal near an absorbance
of zero:
1) start the chart recorder,
2) set the
spectrophotometer absorbance reading to zero,
3) wait until
the baseline stops drifting,
4) set the reading to zero
again.
6.8. Analytical
Procedure
6.8.1. Samples: Immediately before
analyzing, transfer an appropriate aliquot (It is
recommended to use 10 mL of each wipe and bulk and 25 to 50
mL of each air filter sample. Aliquot amounts from air
filter sample solutions can be determined from the air
volume taken. A general rule is to take an aliquot which
will allow a detection limit of at least 0.1 times the PEL)
of the sample solution to a clean B.O.D. bottle containing
enough 10% HNO3 solution to bring
the total volume to 100 mL. The transfer must be done with a
volumetric pipet.
6.8.2. Standards: Immediately
before analyzing, prepare standards according to
instructions listed in Section 6.5.2.
6.8.3. Deliver
5 mL of the 10% SnCl2 solution
with an automatic pipet to a B.O.D. bottle containing a
standard, reagent blank, or sample to be analyzed.
Immediately place the aerator into the solution with the
peristaltic pump operating at full speed.
6.8.4.
Record the maximum absorbance reading and label the signal
produced on the strip chart.
6.8.5. Stop the pump,
remove the B.O.D. bottle from the CV-AAS and stopper it.
Rinse the aerator with DI
H2O and insert it into a
holder in the exhaust vent. Turn the pump on at full speed
until the CV-AAS system is purged of mercury and the
baseline returns to zero.
6.8.6. If the absorbance
reading of a sample is greater than the highest standard
at any time during analysis, immediately
remove the B.O.D. bottle from the CV-AAS. Purge the system
following the procedure listed in Section 6.8.5. Take a
smaller aliquot or dilute the high concentration sample and
re-analyze. Make any necessary sample dilutions with 10%
HNO3 and use the appropriate
dilution factor when calculating results.
6.8.7.
Repeat Sections 6.8.3. through 6.8.5. for each prepared
standard, reagent blank, or sample.
6.9. Analytical Recommendations
6.9.1. It is recommended to analyze the
reagent blank, lowest, and highest standard two or three
times each to check for contamination, reproducibility, and
sensitivity before starting the sample analysis. A 2.0-µg
mercury standard should give a three-quarter to full-scale
deflection on the chart recorder and an absorbance unit
reading of about 0.850 when using the equipment and
conditions specified. The lowest and highest standard should
provide a linear response and the lowest standard should be
at least two to three times the blank signal.
6.9.2.
It is also recommended to analyze an entire series of
standards (including the reagent blank) at the beginning and
end of the sample analysis to ensure standard readings are
reproducible. As a general guideline, standard readings
should be within ±10% throughout the analysis.
6.9.3.
A standard near the concentration range of the samples
should be analyzed after every four to five
samples.
6.9.4. Quality control (QC) samples should
be prepared and analyzed using the same matrix and
analytical conditions as the samples. If possible, the QC
samples should be generated from an independent
source.
6.9.5. Approximately 10% of the samples
should be reanalyzed.
7. Calculations
7.1. Use a least squares regression
program to plot a concentration-response curve of peak
absorbance versus the amount (µg) of mercury in each
standard.
7.2. Determine the amount (µg) of mercury,
A, corresponding to the peak absorbance in each analyzed
sample aliquot from this curve.
7.3. Calculate the
total amount (µg) of mercury, W, in each sample:
| W = |
(A)
(sample volume, mL) (DF)
(aliquot, mL) |
Where:
DF = Dilution Factor (if none, DF =
1)
7.4. Calculate the total concentration of mercury
in each sample using the appropriate
equation:
| Mercury
mg/m3 |
= |
|
(Air
Samples) |
|
| Total
Mercury |
= |
W - Wb |
(Wipe
Samples) |
|
| Mercury %
(w/w) |
= |
(W)
(100%)
(sample wt, mg) (1,000
µg/mg) | |
(Bulk
Samples) |
| Where: |
|
| Wb |
= |
total µg of mercury in
the blank sample. |
| Sample wt |
= |
aliquot of bulk taken in Section
6.6.1. |
7.5.
Reporting Results
7.5.1. Air sample results are reported
as mg/m3 mercury.
7.5.2.
Wipe sample concentrations are reported as total
micrograms or milligrams mercury.
7.5.3. Bulk
sample results are reported as approximate percent by
weight mercury. Due to differences in sample matrices
between bulks and standards, bulk results are approximate.
8. References
8.1. Hatch, W.R. and W.L Ott:
Determination of Submicrogram Quantities of Mercury by
Atomic Absorption Spectrophotometry. Anal. Chem.,
1968, 40, 2085-87.
8.2. Occupational Safety
and Health Administration Technical Center: Mercury
Vapor in Workplace Atmospheres (OSHA-SLTC Method No.
ID-140). Salt Lake City, UT. Revised 1991.
8.3.
National Institute for Occupational Safety and
Health: Criteria for a Recommended Standard --
Occupational Exposure to Inorganic Mercury (DHEW/NIOSH
Pub. No. HSM-73-11024). Cincinnati, OH:
National Institute for Occupational Safety and Health,
1973.
8.4. Windholz, M., ed.: The Merck
Index. 10th ed. Rahway, NJ: Merck & Co. Inc.,
1983.
8.5. Sax, N.I. and R.J. Lewis Sr., ed.:
Hawley's Condensed Chemical Dictionary, 11th ed.; New
York: Van Nostrand Reinhold Co., 1987.
8.6.
Occupational Safety and Health Administration Analytical
Laboratory: Detection Limit Study for Mercury Cold
Vapor Analysis by C. Merrell. Salt Lake City, UT. 1987
(unpublished).
8.7. Occupational Safety and Health
Administration Analytical Laboratory: Quality Control
Data - Mercury-Spiked Filter Cold Vapor Analysis by B.
Babcock. Salt Lake City, UT. 1987 (unpublished).
8.8.
Occupatioml Safety and Health Administration Analytical
Laboratory: Phenyl Mercuric Acetate (PMA)
Procedure by S. Edwards. Salt Lake City, UT. 1981
(unpublished).
Cold vapor-atomic
Absorption Spectrophotometer for Mercury Analysis
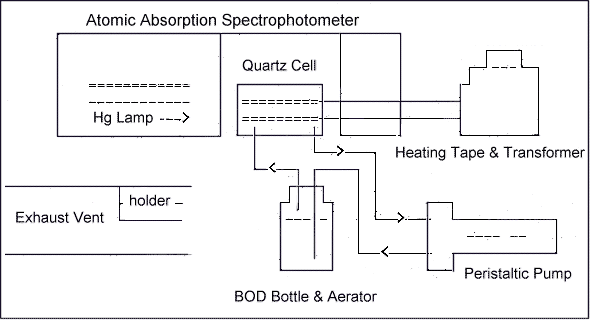
Figure 1
|

