| Method Number: | ID-166 | |||
| Matrix: | Air | |||
| ACGIH Threshold Limit Value (TLV): | 50 ppm Time Weighted Average (TWA) | |||
| NIOSH Recommended Exposure Limit (REL): | 25 ppm TWA | |||
| OSHA PEL: | None | |||
| Collection Device: | Landauer or other nitrous oxide passive monitor | |||
| Recommended Minimum dose: | 25 ppm-hrs (claimed by manufacturer) | |||
| Working range: | 25 to 9,000 ppm-hrs (claimed by manufacturer) | |||
| Analytical Procedure: | Samples are thermally desorbed and analyzed by infra-red (IR) spectroscopy by the manufacturer. | |||
| Validation Level*: | 10 to 500 ppm | |||
| Detection Limit | ||||
| Quantitative: | 2 µg (claimed by manufacturer) | |||
| Precision and Accuracy | ||||
| Validation Range,
ppm: CV2: Average recovery, %: |
| |||
| CV(pooled) (12.5 to 500 ppm*): | 0.084 (includes 3 ranges pooled above) | |||
| Bias (12.5 to 500 ppm*): | -0.047 (includes 3 ranges pooled above) | |||
| Overall Error: | ±21.5% (12.5 to 500 ppm*) | |||
| Method Classification: | Validated Method | |||
| Chemist: | James C. Ku | |||
| Industrial Hygienist (field data): | Ed Zimowski (OSHA Health Response Team) | |||
| Date (date revised): | 1985 (May, 1994) | |||
| * | @ 25 °C and 760 mmHg |
| ** | Normally the range will only include 0.5, 1, and 2 times PEL; due to large anticipated range of air concentration results and two different exposure limits (50 and 25 ppm) data is included for a wider range of concentrations. |
OSHA Salt Lake Technical Center
Salt Lake City, Utah
for descriptive use only and do not constitute endorsements by
oxide, similar products from other sources can be substituted. Substitution is allowed
provided validation procedures are conducted to determine sampling and analytical efficacy.
1. Introduction
This method describes the sample collection of airborne nitrous oxide (N2O). Passive samples are taken in the breathing zone of workplace personnel, and analysis is performed using thermal desorption/infrared (IR) spectroscopy. The analysis is performed by the manufacturer of the passive dosimeter at the manufacturer's laboratory. [COMMENT1]
- 1.1. The effects of occupational exposure to
N2O are still uncertain. However, there is
sufficient concern to warrant positive steps to control the airborne
levels of N2O in workplaces, such as
medical, dental and veterinary facilities. The National Institute for
Occupational Safety and Health (NIOSH) apparently recommended a Time
Weighted Average (TWA) concentration of 50 ppm when
N2O is used in dental offices, and 25 ppm
when it is used during anesthetic administration (5.1.). This has been
recently clarified as a 25 ppm TWA REL (5.2.) regardless of site. The
reference to 50 ppm was considering what is feasible using engineering
and other controls in dental offices. Scavenger systems in dental
operatories are less efficient in capturing
N2O due to the oral dental operations being
performed.
In the past, N2O, the most commonly used anesthetic agent, could only be sampled by gas bags, evacuated containers, or syringes and subsequently analyzed by IR (5.1.). Recently, R.S. Landauer, Jr. and Company (Glenwood, IL) introduced a passive diffusion monitor for N2O. These passive monitors have been evaluated in field studies and were shown to compare favorably with an IR gas analyzer [(5.3.) and also see Addendum to this method]. Other passive systems are available for N2O and can be substituted in this method provided validation procedures are conducted to assure precise and accurate determinations are possible. NIOSH has published a very rigorous procedure (5.4.) for validating passive monitors after this evaluation was performed.
An OSHA laboratory evaluation was conducted over a broad range of N2O concentrations to determine if the Landauer passive monitor is acceptable for use by OSHA industrial hygienists for determining N2O in work environments. In addition, a short field evaluation was performed by the OSHA Health Response Team (see Addendum for further information).
1.2. Principle of the Method
The Landauer nitrous oxide monitor (NITROX®) is a diffusion type air monitoring badge assembly worn in the breathing zone of personnel to evaluate potential exposure to N2O gas. Nitrous oxide gas is adsorbed on the selected adsorbent material (molecular sieve), sent to the laboratory and thermally desorbed and analyzed by the manufacturer using IR. Both an active cartridge sample collected by drawing air through the cartridge with a calibrated sampling pump, (referred to as "active samples" in this report), and a passive monitor sample which requires no sampling pump to collect the sample (referred to as "passive samples" in this report) were taken. Both use the same proprietary sorbent material. This report presents data on both sampling procedures.
1.3. Advantages and Disadvantages
- 1.3.1. This method has adequate sensitivity for measuring
workplace atmosphere concentrations of
N2O.
1.3.2. The sampling procedure for this method involves no liquid and mechanical pumps. A somewhat bulky direct-reading instrument is not used and pre- and post-calibration is not necessary.
1.3.3. One disadvantage is the requirement that the monitor is analyzed at the manufacturer's laboratory, which does not allow for immediate results as given by a direct-reading instrument. Quality control is dependent mainly on the manufacturer; this makes it difficult for those laboratories which prefer to conduct their own quality control program. It is recommended that users occasionally prepare spiked samples to assure adequate quality control.
1.4. Method Performance
A synopsis of the method performance is presented below. Further information can be found in Section 4.
- 1.4.1. This method was validated over the concentration range of
10 to 500 ppm.
1.4.2. The quantitative detection limit was 2 µg N2O (manufacturer claim).
1.4.3. The validation ranges, the coefficients of variation (CV2), and average recoveries are:
| Validation Range, ppm: | 12.5 to 500 | 25 to 110 | 25 to 500 |
| CV2(pooled): | 0.135 | 0.093 | 0.080 |
| Average recovery, %: | 98.7 | 93.1 | 103.1 |
Using data derived from 5 sample sets (12.5, 25, 50, 110 ppm, and one 500 ppm set) the total pooled CV2, Bias, and Overall Error are:
| CV(pooled) = 0.084; | Bias = -0.047; | Overall Error = ±21.5% |
1.4.4. Samples can be stored at ambient (20 to 25 °C) temperature on a lab bench for a period of 30 days. Results show the mean sample recovery after 30 days of storage was within ±10% of results at Day 2.
1.5. Interferences
No known interferences were reported by the manufacturer.
1.6. Uses and Sources (5.5.)
- byproduct of nylon production (5.6.)
anesthetic in dentistry and surgery
propellent gas in food aerosols and whipped cream
leak detection
1.7. Physical and Chemical Properties (5.7.)
Nitrous oxide exists as colorless, nonexplosive, nonflammable gas at room temperature. The gas promotes combustion similar to oxygen and has a slightly sweet odor and taste. Ambient concentrations of N2O are produced by decomposition of nitrogen compounds found in the soil and are approximately 0.25 ppm.
| Nitrous Oxide (CAS No. 10024-97-2) | |
| Chemical formula | N2O |
| Molecular weight | 44.02 |
| Melting point | -90.81 °C |
| Boiling point | -88.5 °C |
| Density | 1.53 times that of air |
| Solubility | Soluble in alcohol, water, ether, oils, sulfuric acid |
| Flammability | Nonflammable, supports combustion |
| Synonyms | Laughing gas, nitrogen oxide*, dinitrogen monoxide, hyponitrous acid anhydride, factitous air. |
* Unfortunately, nitrous oxide has been confused with nitric oxide (NO) resulting in disastrous consequences.
1.8. Toxicology (5.7.)
Information listed within this section is a synopsis of current knowledge of the physiological effects of N2O and is not intended to be used as a basis for OSHA policy. More information regarding toxicity can be found in reference 5.7.
Previously, N2O was considered a simple asphyxiant. Current data indicates a causal relationship in producing in bone marrow depression and granulocytopenia, potential teratogenicity and spontaneous abortion. Nitrous oxide-induced bone marrow depression and granulocytopenia appear reversible. Nitrous oxide has been implicated in also producing neurotoxic, hepatic, and renal effects. Neurotoxic effects such as numbness, tingling, and weakness have been demonstrated in early studies and NIOSH has based their 25 ppm REL on decreased audiovisual performance noted when subjects were exposed to 50 ppm N2O (5.1.). Biological systems most susceptible to N2O toxicity are reproductive, hematologic, and the nervous system. Evidence for physiological risk to indicate short-term exposures warrant STEL or Ceiling Limits has not been adequately demonstrated (5.7.).
2. Sampling
- 2.1. Follow the manufacturer's instructions regarding usage. These
should be provided to every user. Record sample start and stop times,
and sample identification numbers.
2.2. A pump is not used and sampling commences as soon as the upper cap is removed. Remove the upper cap and place in the breathing zone of the employee.
Note: The manufacturer has indicated that the monitor can be placed under the top layer of surgical gowns or scrubs without affecting the sampling uptake. Losses of approximately 10% have been noted under three layers of clothing placed in test chambers. This method has not evaluated the ability for the sampler to perform under such conditions; however, there is obviously a potential for bloodborne pathogen contamination of the dosimeter during sampling. Side-by-side sampling (placing a monitor inside and outside different surgical gowns for a statistically significant number of employees) during actual work procedures may need to be performed to assure the sample uptake rate is not adversely affected.

Individuals conducting sampling in areas where bloodborne pathogen
exposure is possible will refer to OSHA Instruction CPL
2.3. Plan on sampling for a period of up to 8 hours to determine TWA exposures. Remove the dosimeter from the employee and place the cap tightly on the NITROX® Dosimeter to cease sampling.
2.4. Write down any pertinent sampling information on the OSHA 91A and request analysis for nitrous oxide (N2O).
2.5. Wrap sample seals lengthwise on each dosimeter tube after sampling and ship samples and paperwork to the OSHA Salt Lake Technical Center (SLTC) for sample tracking. Compliance officers for OSHA do not have to ship the dosimeters to Landauer, Inc. The OSHA-SLTC will track the samples and relay the results immediately after receipt to the compliance officer.
2.6. Always include a blank dosimeter with every set of N2O samples.
2.7. Bulk and wipe samples are inappropriate for this method. Leak testing of the delivery systems for N2O should be conducted using an appropriate direct-reading IR or other suitable instrument.
3. Analysis
Sample analysis is proprietary and is conducted by the manufacturer. Samples are thermally desorbed and analyzed by IR spectroscopy. Results are reported back to the person submitting samples as ppm TWA and ppm-hours N2O.
4. Backup Data
This method has been validated for a concentration range of 10 to 500 ppm N2O. The method validation was conducted near the ACGIH TLV of 50 ppm N2O and the NIOSH REL of 25 ppm. In addition, a high concentration (500 ppm) expected in some operations was also examined.
The validation consisted of the following experiments and discussion:
- 1. A preliminary analysis of 20 active samples was performed (10
samples each at 0.5 × and 1 × TWA TLV) to evaluate and to check the
generation system.
2. A sampling and analysis of 70 active samples (mixed concentration from 10 to 500 ppm N2O) collected from dynamically generated test atmospheres at 60% RH to determine the precision and accuracy of the manufacturer.
3. A sampling and analysis of 38 passive monitors (mixed concentration from 10 to 500 ppm N2O) collected from dynamically generated test atmospheres at 60% RH to determine precision and accuracy analyzed by the manufacturer.
4. An evaluation of storage stability at room (20 to 25 °C) temperature for 20 collected samples.
5. An evaluation of reverse diffusion for the monitors.
6. High humidity tests for Landauer active cartridges and Landauer passive monitors.
7. Comparison of sampling methods: Active cartridges versus passive monitors.
8. Summary.
Evaluation Protocol
Tests were performed on a minimum of six monitors for each concentration level evaluated at relative humidities of 60% and a temperature of 25 °C, which were considered typical environmental conditions for operating rooms. The evaluation consisted of the following major experiments: precision and accuracy, storage stability, reverse diffusion, high humidity and comparison of methods.
- 4.1. Preparation and Monitoring of Nitrous Oxide Concentrations
- 4.1.1. Concentration of cylinder of neat nitrous oxide:
A cylinder of neat N2O was used for these experiments. The concentration of N2O had been certified by the manufacturer as USP grade N2O. This concentration was used as the "true" cylinder concentration, and was used to prepare known concentrations of N2O.
4.1.2. Samples from the cylinder of N2O (spikes were delivered to molecular sieve) were sent to Landauer Company for analysis and verification of the cylinder concentration. Landauer reported a concentration of 100.2% N2O. (Refer to Table 1)
4.2. Generation Apparatus:
- 4.2.1 The generation apparatus used provided a means of
generating dynamic test atmospheres. The block diagram of the major
components in the dynamic generation system is shown in Figure 1.
The system consists of six essential elements:
- (1) a flow-temperature-humidity control system,
(2) a N2O gas generating system (including a mass flow controller),
(3) a mixing chamber,
(4) an active sampling manifold,
(5) a monitor exposure chamber and
(6) an IR analyzer used as a continuous monitor.
A detailed description of the generation system can be found in references 5.8. and 5.9.
4.2.2 Preliminary experiments were conducted with two sets of active samplers in order to determine if the generation system was working properly. These results are reported in Table 2.
4.3. On-line Monitoring of Gas Concentrations
A MIRAN 103 gas analyzer was used as an on-line continuous monitor of generated N2O gas concentrations. Calibration of the analyzer was performed by employing a closed-loop circulating system following the procedure described in the MIRAN 103 operating manual (5.10.). The calibration curve was obtained by plotting the N2O concentration in ppm vs. peak height in mm (from the recorder connected to the IR. The IR used had a 4.5 micron nitrous oxide filter).
4.4. Sampling and Analysis
- 4.4.1. Sampling: Known (theoretical) concentrations of
N2O were prepared by dilution of the
certified N2O with purified compressed air
using mass flow controllers, with a manufacturer's reported accuracy
of ±2%. These theoretical concentrations (as calculated from the
flow dilutions) were also independently verified by the manufacturer
(R.S. Landauer, Jr. and Company) from active samples submitted for
analysis. Good agreement was found between the active and
theoretical sampling results (Table 3).
4.4.2. Analysis: All samples, (active cartridges and passive monitors) were collected, sealed and sent to the manufacturer for analysis.
4.5. Precision and Accuracy
The precision and accuracy data were obtained by exposing groups of at least six samples (both active cartridges and passive monitors) to known concentrations of N2O. A broad range of concentrations and a variety of sampling periods were used. These test results are presented in Tables 3 and 4. The coefficients of variation, CV2, and the average recoveries for exposed monitors for three ranges of the NIOSH and ACGIH proposed exposure limits from (1) 0.5 to 20 times the 25 ppm limit, (2) 0.5 to 2 times the 50 ppm limit, and (3) 0.5 to 10 times the 50 ppm limit. (Note: The 20 times concentrations were anticipated in actual workplace environments). These results were compared to active samplers taken side-by-side and recoveries were determined based on the active sampler results.
| CV2, % | Avg. Recovery, % | |||
| Conc. Range, ppm* | Active | Passive | Active | Passive |
| 12.50 - 500.00 | 4.9 | 13.5 | 99.3 | 98.7 |
| 25.00 - 110.00 | 2.2 | 9.3 | 99.0 | 93.1 |
| 25.00 - 500.00 | 5.1 | 8.0 | 101.8 | 103.1 |
| *(@ NTP = 25 °C, & 760 mmHg) | ||||
Using data derived from 5 sample sets (12.5, 25, 50, 110 ppm, and one 500 ppm set) the total pooled CV2, Bias, and Overall Error are:
| CV(pooled) = 0.084; | Bias = -0.047; | Overall Error = ±21.5% |
4.6. Storage Stability
A study was conducted to assess the stability of the passive monitors when stored at laboratory ambient temperature. Four sets, each containing four to six passive monitors, were exposed to a N2O concentration of 25 ppm. Each set was analyzed by the Landauer Company after various periods of storage ( 2, 7, 15, and 30 days). Table 5 presents the results of this study which according to the manufacturer's analysis shows that the mean of samples stored for 30 days are within ±10% of the mean of the passive monitors that were analyzed after 2 days.
4.7. Reverse Diffusion
Two sets of passive monitors were used for a reverse-diffusion study. The first set of six monitors was exposed at 500 ppm N2O gas, 60% RH, and 25 °C for 2 hours. The second set of six monitors was exposed under the same conditions as the first set except that the monitors were exposed continuously for another four hours in air which contained no N2O. As shown in Table 6, the difference between the means of the two sets in terms of ppm-hour is less than 8%. These results indicate that reverse diffusion would not be a significant problem for at least an 8-hour sampling period.
4.8. High Humidity
An experiment was conducted to determine if high humidity (90% RH, 25 °C) had an effect on sample collection by the passive monitors.
- 4.8.1. Procedure: Because the primary and back-up
adsorbent cells in the passive monitor contained the same adsorbent
material as the active cartridge, two separate tests were performed
for active and passive samples.
4.8.2. Four sets of active samples were taken for a variety of sampling times at a flowrate of between 10 and 20 cc/min. Each set contained five samples.
4.8.3. Six passive monitors were simultaneously exposed for 7 hours according to the procedure described in Appendix A.
4.8.4. All active and passive samplers were sent to the manufacturer for analysis.
4.8.5. Results: The results of this high humidity study are shown in Tables 7 and 8 for active and passive samples, respectively. As can be seen, the results of all active samplers indicate that no significant changes are found among them for sampling times of 30, 60, 100, and 120 minutes. Table 8 also shows that passive monitors can accurately and precisely measure known N2O challenges at relative humidities of 60 and 90% and N2O concentrations as high as 500 ppm for an 8-hour shift.
4.9. Comparison of Landauer Active Sampling and Passive Monitor Methods for N2O:
A study was conducted to compare the performance of Landauer active cartridge samplers with that of Landauer passive monitors for determining N2O concentrations at 25 °C and 60% RH. All active cartridges and passive monitors were exposed simultaneously using the same environmental conditions. Table 9 summarizes the data for comparison of these two sampling methods. As shown, the pooled coefficients of variation CV2 (pooled) are 4.9 and 13.5% with average recoveries in terms of percentage (%) over the concentration range studied of 99.3 and 98.7% for active cartridges and passive monitors, respectively.
4.10. Summary and Conclusion:
The Landauer N2O monitors offer significant advantages over traditional methods, particularly in the area of convenience. The monitors show good accuracy and precision except at the lowest concentration of 12 ppm where the mass collected is very close to the detection limit claimed by the manufacturer. The CVs of monitors over concentrations from 25 to 500 ppm are less than ±10%. The average recoveries in comparison with the active cartridges are close to 100%.
The monitors show excellent stability after exposure. There were no significant differences between monitors analyzed after two days (Note: This is the minimum time required to send to the manufacturer for analysis) and monitors analyzed after 30 days. There is no evidence to show that the reverse diffusion and high humidity can affect the performance of the monitor over an 8-hour shift. One disadvantage is the manufacturer requirement to have monitors analyzed at the manufacturer's laboratory.
5. References
- 5.1. National Institute for Occupational Safety and Health
(NIOSH): Criteria for a Recommended Standard - Occupation
Exposure to Waste Anesthetic Gases and Vapors, (DHEW/NIOSH Pub.
No. 77-140 77) Cincinnati, OH: NIOSH, 1977.
5.2. National Institute for Occupational Safety and Health: Recommendations for Occupational Safety and Health - Compendium of Policy Documents and Statements (DHHS/NIOSH Pub. No. 92-100). Cincinnati, OH: NIOSH, 1992.
5.3. Bishop, E.C. and M.A. Hossain: Field Comparison between Two Nitrous Oxide Passive Monitors and Conventional Sampling Methods, Am. Ind. Hyg. Assoc. J. 45: 812 (1984).
5.4. Cassinelli, M.E., R.D. Hull, J.V. Crable, and A.W. Teass: Protocol for the Evaluation of Passive Monitors. In Diffusion Sampling, An Alternative Approach to Workplace Monitoring, edited by A. Berlin, R.H. Brown, and K.J. Saunders. London: Royal Society of Chemistry, 1987. pp. 190-202.
5.5. Sax, N.I. and R.J. Lewis, Sr.: Hawley's Condensed Chemical Dictionary. 11th ed. New York: Van Nostrand Reinhold Co., 1987.
5.6. Thiemens, T.H. and Trogler, W.C.: Nylon production: An unknown source of atmospheric nitrous oxide. Science, 251, 932-934 (1991).
5.7. American Conference of Governmental Industrial Hygienists (ACGIH): Documentation of the Threshold Limit Values and Biological Exposure Indices, 6th ed. Cincinnati, OH: ACGIH, 1991, 445.1-445.4.
5.8. Occupational Safety and Health Administration Analytical Laboratory: Formaldehyde in Workplace Atmospheres ID-102. In OSHA Analytical Methods Manual 1st ed. Cincinnati, OH: American Conference of Governmental Industrial Hygienists (Pub. No. ISBN: 0-936712-66-X), 1985.
5.9. Occupational Safety and Health Administration Analytical Laboratory: Evaluation of 3M Formaldehyde Monitors (Model 3751) by J.C. Ku (Product Evaluation no. ID-139). Salt Lake City, UT:OSHA Analytical Laboratory, 1982.
5.10. Wilks Scientific Corp: Miran 103 (Gas Analyzer) Operating Manual, South Norwalk, CT: Wilks Scientific Corporation, no publication date given.
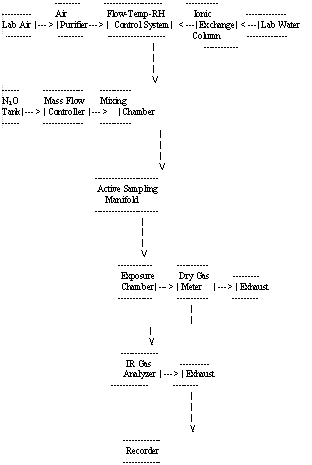
Figure 1. Block Diagram of the Major Components in a Dynamic Generation System
| No. | uL Spiked | ug Taken | ug Found | Statistical Analysis | ||
| S01 | 100 | 156.28 | 155.89 | n | 10 | |
| S02 | 100 | 156.28 | 152.54 | Mean, ug | 154.11 | |
| S03 | 100 | 156.28 | 149.41 | Std Dev, ug | 5.00 | |
| S04 | 100 | 156.28 | 142.86 | CV1, % | 3.2 | |
| S05 | 100 | 156.28 | 157.53 | Purity, % | 98.8 | |
| S06 | 100 | 156.28 | 155.52 | |||
| S07 | 100 | 156.28 | 155.78 | |||
| S08 | 100 | 156.28 | 160.00 | |||
| S09 | 100 | 156.28 | 157.74 | |||
| S10 | 100 | 156.28 | 156.78 | |||
| S11 | 50 | 78.14 | 75.96 | n | 10 | |
| S12 | 50 | 78.14 | 78.00 | Mean, ug | 77.98 | |
| S13 | 50 | 78.14 | 71.46 | Std Dev, ug | 3.12 | |
| S14 | 50 | 78.14 | 78.12 | CV1, % | 4.0 | |
| S15 | 50 | 78.14 | 80.64 | Purity, % | 99.8 | |
| S16 | 50 | 78.14 | 77.53 | |||
| S17 | 50 | 78.14 | 76.17 | |||
| S18 | 50 | 78.14 | 79.32 | |||
| S19 | 50 | 78.14 | 79.39 | |||
| S20 | 50 | 78.14 | 83.16 | |||
| S21 | 250 | 392.44 | 395.21 | n | 4 | |
| S22 | 250 | 392.44 | 414.38 | Mean, ug | 395.61 | |
| S23 | 250 | 392.44 | 376.64 | Std Dev, ug | 15.41 | |
| S24 | 250 | 392.44 | 396.21 | CV1, % | 3.9 | |
| S25 | 250 | 392.44 | LIA | Purity, % | 100.8 | |
| S26 | 100 | 156.97 | 163.76 | n | 5 | |
| S27 | 100 | 156.97 | 162.85 | Mean, ug | 160.47 | |
| S28 | 100 | 156.97 | 160.99 | Std Dev, ug | 3.03 | |
| S29 | 100 | 156.97 | 156.70 | CV1, % | 1.9 | |
| S30 | 100 | 156.97 | 158.07 | Purity, % | 102.2 | |
| S31 | 50 | 78.02 | 78.64 | n | 10 | |
| S32 | 50 | 78.02 | 81.11 | Mean, ug | 77.49 | |
| S33 | 50 | 78.02 | 80.26 | Std Dev, % | 3.25 | |
| S34 | 50 | 78.02 | 80.59 | CV1, % | 4.2 | |
| S35 | 50 | 78.02 | 79.32 | Purity, % | 99.3 | |
| S36 | 50 | 78.02 | 75.00 | |||
| S37 | 50 | 78.02 | 74.05 | CV1(pooled) | 3.7% | |
| S38 | 50 | 78.02 | 79.43 | Av. Purity | 100.2% | |
| S39 | 50 | 78.02 | 74.26 | |||
| S40 | 50 | 78.02 | 72.23 | |||
LIA = Lost in analysis
Preliminary Experiments -
Checking the Generation System Using
Landauer Active Sampling Cartridges
| Sample | Air Vol | Mass Found | N2O Concentration | Statistical | ||
| No. | L | ug | mg/m3 | ppm** | Analysis | |
| G01 | 0.926 | 70.76 | 76.41 | 49.11 | n | 7 |
| G02 | 0.838 | 62.52 | 74.61 | 47.95 | Mean, ppm | 46.60 |
| G03 | 1.132 | 86.31 | 76.25 | 49.01 | Std Dev, ppm | 2.50 |
| G04 | 1.227 | 84.88 | 69.18 | 44.46 | CV2, % | 5.37 |
| G05 | 0.892 | Lost | In | Analysis | Theor. ppm** | 50.00 |
| G06 | 1.395 | Lost | In | Analysis | ||
| G07 | 1.266 | 86.22 | 68.10 | 43.77 | ||
| G08 | 1.722 | 129.17 | 74.97 | 48.19 | ||
| G09 | 1.835 | 230.67 | 125.71* | 80.79* | ||
| G10 | 1.328 | 90.28 | 67.98 | 43.69 | ||
| G11 | 0.925 | 60.49 | 65.39* | 42.03* | n | 8 |
| G12 | 0.840 | 57.12 | 68.00* | 43.71* | Mean, ppm | 25.55 |
| G13 | 1.134 | 46.96 | 41.41 | 26.62 | Std Dev, ppm | 0.83 |
| G14 | 1.220 | 47.27 | 38.75 | 24.90 | CV2, % | 3.26 |
| G15 | 0.869 | 33.64 | 38.71 | 24.88 | Theor, ppm** | 25.00 |
| G16 | 1.386 | 55.33 | 39.92 | 25.66 | ||
| G17 | 1.255 | 49.89 | 39.75 | 25.55 | ||
| G18 | 1.697 | 71.23 | 41.97 | 26.98 | ||
| G19 | 1.850 | 71.86 | 38.84 | 24.97 | ||
| G20 | 1.295 | 50.05 | 38.65 | 24.84 | ||
* Excluded from statistical analysis
** (@ NTP = 25 °C & 760 mmHg)
Precision and Accuracy
for
Landauer N2O Active Sampling Cartridges
| Sample | Air Vol | Mass Found | N2O Concentration | Statistical | ||
| No. | L | ug | mg/m3 | ppm** | Analysis | |
| GA21 | 1.087 | 182.52 | 167.91 | 107.11 | n | 10 |
| GA22 | 0.974 | 168.84 | 173.34 | 110.59 | Mean, ppm | 109.52 |
| GA23 | 1.480 | 259.09 | 175.06 | 111.68 | Std Dev, ppm | 1.79 |
| GA24 | 1.619 | 276.27 | 170.64 | 108.86 | CV2, % | 1.63 |
| GA25 | 0.948 | 164.83 | 173.87 | 110.91 | Recovery, % | 99.6 |
| GA26 | 0.924 | 158.07 | 171.07 | 109.13 | Known ppm*** | 110.00 |
| GA27 | 0.826 | 141.31 | 171.08 | 109.14 | ||
| GA28 | 1.255 | 213.18 | 169.86 | 108.36 | ||
| GA29 | 1.369 | 229.90 | 167.93 | 107.13 | ||
| GA30 | 0.813 | 143.06 | 175.97 | 112.26 | ||
| GA31 | 1.380 | 100.25 | 72.64 | 46.84 | n | 10 |
| GA32 | 1.248 | 89.30 | 71.55 | 46.14 | Mean, ppm | 46.21 |
| GA33 | 1.894 | 132.55 | 69.98 | 45.12 | Std Dev, ppm | 1.15 |
| GA34 | 2.076 | 145.29 | 69.99 | 45.12 | CV2, % | 2.50 |
| GA35 | 1.186 | 86.93 | 73.30 | 47.26 | Recovery, % | 92.4 |
| GA36 | 1.380 | 103.13 | 74.73 | 48.18 | Known ppm*** | 50.00 |
| GA37 | 1.248 | 91.26 | 73.13 | 47.15 | ||
| GA38 | 1.886 | 133.06 | 70.55 | 45.49 | ||
| GA39 | 2.071 | 143.07 | 69.08 | 44.54 | ||
| GA40 | 1.199 | 85.94 | 71.68 | 46.21 | ||
| GA41 | 1.855 | 30.90 | 16.66 | 10.66 | n | 10 |
| GA42 | 1.666 | 29.07 | 17.45 | 11.17 | Mean, ppm | 10.86 |
| GA43 | 2.530 | 41.82 | 16.53 | 10.58 | Std Dev, ppm | 0.34 |
| GA44 | 2.770 | 46.00 | 16.61 | 10.63 | CV2, % | 3.10 |
| GA45 | 1.590 | 26.35 | 16.57 | 10.60 | Recovery, % | 86.9 |
| GA46 | 1.862 | 31.79 | 17.07 | 10.92 | Known ppm*** | 12.50 |
| GA47 | 1.665 | 28.38 | 17.05 | 10.91 | ||
| GA48 | 2.534 | 42.47 | 16.76 | 10.72 | ||
| GA49 | 2.766 | 46.24 | 16.72 | 10.70 | ||
| GA50 | 1.615 | 29.42 | 18.22 | 11.66 | ||
| GA51 | 1.462 | 56.71 | 38.79 | 24.97 | n | 10 |
| GA52 | 1.312 | 51.87 | 39.54 | 25.45 | Mean, ppm | 25.25 |
| GA53 | 1.994 | 78.39 | 39.31 | 25.31 | Std Dev, ppm | 0.51 |
| GA54 | 2.184 | 84.56 | 38.72 | 24.93 | CV2, % | 2.00 |
| GA55 | 1.261 | 50.01 | 39.66 | 25.53 | Recovery, % | 101 |
| GA56 | 1.474 | 57.61 | 39.08 | 25.16 | Known ppm*** | 25.00 |
| GA57 | 1.313 | 49.30 | 37.55 | 24.17 | ||
| GA58 | 1.990 | 79.98 | 40.19 | 25.87 | ||
| GA59 | 2.176 | 85.16 | 39.14 | 25.19 | ||
| GA60 | 1.271 | 51.18 | 40.27 | 25.92 | ||
| GA61 | 1.868 | 75.39 | 40.36 | 25.82 | n | 10 |
| GA62 | 1.663 | 69.32 | 41.68 | 26.67 | Mean, ppm | 26.30 |
| GA63 | 2.535 | 104.86 | 41.36 | 26.47 | Std Dev, ppm | 0.63 |
| GA64 | 2.761 | 115.33 | 41.77 | 26.73 | CV2, % | 2.40 |
| GA65 | 1.612 | 68.20 | 42.31 | 27.07 | Recovery, % | 105 |
| GA66 | 1.876 | 75.36 | 40.17 | 25.70 | Known ppm*** | 25.00 |
| GA67 | 1.661 | 69.49 | 41.84 | 26.77 | ||
| GA68 | 2.520 | 101.02 | 40.09 | 25.65 | ||
| GA69 | 2.745 | 108.27 | 39.44 | 25.24 | ||
| GA70 | 1.589 | 66.66 | 41.95 | 26.84 | ||
| GA71 | 0.449 | 384.19 | 855.66 | 554.23 | n | 9 |
| GA72 | 0.380 | 339.62 | 893.74 | 578.90 | Mean, ppm | 545.24 |
| GA73 | 0.571 | Lost | In | Analysis | Std Dev, ppm | 20.81 |
| GA74 | 0.631 | 539.83 | 855.52 | 554.14 | CV2, % | 3.82 |
| GA75 | 0.420 | 346.56 | 825.14 | 534.47 | Recovery, % | 109 |
| GA76 | 0.447 | 366.97 | 820.96 | 531.76 | Known ppm*** | 500.00 |
| GA77 | 0.380 | 335.39 | 882.61 | 571.69 | ||
| GA78 | 0.566 | 470.86 | 831.91 | 538.85 | ||
| GA79 | 0.630 | 505.07 | 801.70 | 519.28 | ||
| GA80 | 0.415 | 335.62 | 808.72 | 523.83 | ||
| GA81 | 0.887 | 749.03 | 844.45 | 546.14 | n | 19 |
| GA82 | 0.762 | 618.65 | 811.88 | 525.07 | Mean, ppm | 516.59 |
| GA83 | 1.140 | 923.64 | 810.21 | 523.99 | Std Dev,ppm | 41.36 |
| GA84 | 1.264 | 1,063.36 | 841.27 | 544.08 | CV2, % | 8.01 |
| GA85 | 0.824 | 642.26 | 779.44 | 504.09 | Recovery, % | 103 |
| GA86 | 0.448 | < 1* | - | - | Known ppm*** | 500.00 |
| GA87 | 0.381 | 330.03 | 866.22 | 560.22 | ||
| GA88 | 0.570 | 351.88 | 617.33 | 399.25 | CV2(Pooled),% | 4.64 |
| GA89 | 0.630 | 527.51 | 837.32 | 541.52 | Avg.Recovery % | 99.6 |
| GA90 | 0.410 | 343.36 | 837.26 | 541.62 | ||
| GA91 | 1.800 | 1,294.23 | 719.02 | 465.01 | ||
| GA92 | 1.525 | 1,178.50 | 772.79 | 499.79 | ||
| GA93 | 2.287 | 2,093.73 | 915.49 | 592.08 | ||
| GA94 | 2.522 | 2,095.36 | 830.83 | 537.33 | ||
| GA95 | 1.635 | 1,264.97 | 773.68 | 500.37 | ||
| GA96 | 1.499 | 1,104.59 | 736.88 | 476.57 | ||
| GA97 | 1.270 | 1,031.42 | 812.14 | 525.24 | ||
| GA98 | 1.902 | 1,513.91 | 795.96 | 514.77 | ||
| GA99 | 2.101 | 1,719.76 | 818.54 | 529.38 | ||
| GA100 | 1,356 | 1,024.56 | 755.57 | 488.66 | ||
* Excluded from statistical analysis
** The environmental conditions of 90% RH and 25 °C were used.
*** (@ NTP)
Precision and Accuracy
for
Landauer N2O Passive Monitors
(60% RH and 25 °C)
| Known | Samp Time | Sample | N2O Concentration | Statistical | ||
| ppm** | Min | No. | ug | ppm** | Analysis | |
| 12.50 | 420 | GP25 | 3.21 | 7.28 | n | 6 |
| GP26 | 3.13 | 7.10 | Mean, ppm | 9.58 | ||
| GP27 | 5.50 | 12.47 | Std Dev, ppm | 2.72 | ||
| GP28 | 4.11 | 9.32 | CV2, % | 28.3 | ||
| GP29 | 3.50 | 7.94 | Recovery, % | 88.2 | ||
| GP30 | 5.90 | 13.38 | ||||
| 25.00 | 420 | GP41 | 11.51 | 26.11 | n | 6 |
| GP42 | 9.16* | 20.78 | Mean, ppm | 23.44 | ||
| GP43 | 11.59 | 26.29 | Std Dev, ppm | 2.40 | ||
| GP44 | 9.23* | 20.93 | CV2, % | 10.20 | ||
| GP45 | 10.34 | 23.45 | Recovery, % | 89.0 | ||
| GP46 | 10.18 | 23.09 | ||||
| 50.00 | 420 | GP19 | 22.68 | 51.84 | n | 6 |
| GP20 | 17.97 | 41.08 | Mean, ppm | 45.60 | ||
| GP21 | 17.00 | 38.86 | Std Dev, ppm | 5.47 | ||
| GP22 | 19.49 | 44.55 | CV2, % | 11.99 | ||
| GP23 | 22.83 | 52.19 | Recovery, % | 98.7 | ||
| GP24 | 19.72 | 45.10 | ||||
| 110.00 | 300 | GP13 | 34.36 | 105.68 | n | 6 |
| GP14 | 34.52 | 106.17 | Mean, ppm | 103.89 | ||
| GP15 | 32.69 | 100.54 | Std Dev, ppm | 3.54 | ||
| GP16 | 35.08 | 107.89 | CV2, % | 3.40 | ||
| GP17 | 33.92 | 104.32 | Recovery, % | 94.9 | ||
| 500.00 | 120 | GP51 | 86.26 | 622.50 | n | 5 |
| GP52 | 79.16 | 571.26 | Mean, ppm | 610.79 | ||
| GP53 | 86.40 | 623.51 | Std Dev, ppm | 24.66 | ||
| GP54 | 87.78 | 633.46 | CV2, % | 4.00 | ||
| GP55 | Lost In Analysis | Recovery, % | 112.00 | |||
| GP56 | 83.59 | 603.23 | ||||
| 500.00 | 420 | GP63 | 248.88 | 570.63 | n | 8 |
| GP64 | 211.05 | 483.90 | Mean, ppm | 569.30 | ||
| GP65 | 249.34 | 571.69 | Std Dev, ppm | 36.45 | ||
| GP66 | 256.96 | 589.16 | CV2, % | 6.40 | ||
| GP67 | 252.45 | 578.82 | Recovery, % | 110.2 | ||
| GP68 | 254.77 | 584.14 | ||||
| GP69 | 248.93 | 570.75 | ||||
| GP70 | 263.99 | 605.28 | ||||
| * Some N2O found
in backup cartridge. ** (@ NTP) | ||||||
| Concentration Range, ppm** | CV2(pooled), % | Avg. Recovery, % |
| 12.50 - 500.00 | 13.5 | 98.7 |
| 25.00 - 110.00 | 9.3 | 93.1 |
| 25.00 - 500.00 | 8.0 | 103.1 |
* (@ NTP)
Storage Stability
for
Landauer N2O passive Monitors
| Known | Sample Age | Sample | N2O Found | Statistical | |
| ppm** | Days | No. | ppm | Analysis | |
| 25.00 | 2 | GP41 | 26.11 | n | 6 |
| GP42 | 20.78* | Mean, ppm | 23.44 | ||
| GP43 | 26.29 | Std Dev, ppm | 2.40 | ||
| GP44 | 20.93* | CV2, % | 10.20 | ||
| GP45 | 23.45 | ||||
| GP46 | 23.09 | ||||
| 25.00 | 7 | GP31 | 25.02 | n | 5 |
| GP32 | 24.74 | Mean, ppm | 26.64 | ||
| GP33 | 30.75 | Std Dev, ppm | 2.53 | ||
| GP34 | 25.29 | CV2, % | 9.50 | ||
| GP35 | 27.41 | ||||
| 25.00 | 15 | GP36 | 21.80 | n | 5 |
| GP37 | 25.04 | Mean, ppm | 25.16 | ||
| GP38 | 29.58 | Std Dev, ppm | 3.37 | ||
| GP39 | 22.02 | CV2, % | 13.4 | ||
| GP40 | 27.34 | ||||
| 25.00 | 30 | GP47 | 22.75 | n | 4 |
| GP48 | 22.75 | Mean, ppm | 21.58 | ||
| GP49 | 20.53 | Std Dev, ppm | 1.35 | ||
| GP50 | 20.30 | CV2, % | 6.26 | ||
| * Some N2O found
in backup cartridges. ** (@ NTP) | |||||
Reverse Diffusion
for
Landauer N2O Passive Monitors
| Exposure Time | Sample | -----N2O Concentration----- | Statistical | |||||||
| Hrs. | No. | ug | ppm* | ppm-hour | Analysis | |||||
| 2 | GP51 | 86.26 | 622.50 | 1,245.0 | n | 5 | ||||
| GP52 | 79.16 | 571.26 | 1,142.5 | Mean, ppm-hrs | 1,221.6 | |||||
| GP53 | 86.40 | 623.51 | 1,247.0 | Std Dev, ppm-hrs | 49.3 | |||||
| GP54 | 87.78 | 633.46 | 1,266.9 | CV2, % | 4.0 | |||||
| GP55 | Lost | In | Analysis | |||||||
| GP56 | 83.59 | 603.23 | 1,206.5 | |||||||
| 2 + 4 Hrs | GP57 | 67.69 | 178.95 | 1,073.7 | n | 6 | ||||
| "Zero" Air | GP58 | 73.37 | 193.97 | 1,163.8 | Mean, ppm-hrs | 1,146.8 | ||||
| GP59 | 74.68 | 197.43 | 1,184.6 | Std Dev, ppm-hrs | 46.3 | |||||
| GP60 | 73.40 | 194.05 | 1,164.3 | CV2, % | 4.0 | |||||
| GP61 | 69.74 | 184.37 | 1,106.2 | |||||||
| GP62 | 68.59 | 181.33 | 1,188.0 | |||||||
| ||||||||||
* (@ NTP)
High Humidity Test
for
Landauer Active Cartridges
(Known Conc. = 500.00 ppm)
| Sample | Air Vol | Mass Found | N2O Concentration | Statistical | ||
| No. | L | ug | mg/m3 | ppm** | Analysis | |
| GA81 | 0.887 | 749.03 | 844.45 | 546.14 | n | 5 |
| GA82 | 0.762 | 618.65 | 811.88 | 525.07 | Mean, ppm | 528.67 |
| GA83 | 1.140 | 923.64 | 810.21 | 523.99 | Std Dev,ppm | 17.19 |
| GA84 | 1.264 | 1,063.36 | 841.27 | 544.08 | CV2, % | 3.25 |
| GA85 | 0.824 | 642.26 | 779.44 | 504.09 | Recovery, % | 106 |
| GA86 | 0.448 | < 1* | - | - | n | 4 |
| GA87 | 0.381 | 330.03 | 866.22 | 560.22 | Mean, ppm | 510.65 |
| GA88 | 0.570 | 351.88 | 617.33 | 399.25 | Std Dev, ppm | 74.79 |
| GA89 | 0.630 | 527.51 | 837.32 | 541.52 | CV2, % | 14.65 |
| GA90 | 0.410 | 343.36 | 837.26 | 541.62 | Recovery, % | 102 |
| GA91 | 1.800 | 1,294.23 | 719.02 | 465.01 | n | 5 |
| GA92 | 1.525 | 1,178.50 | 772.79 | 499.79 | Mean, ppm | 518.92 |
| GA93 | 2.287 | 2,093.73 | 915.49 | 592.08 | Std Dev, ppm | 48.24 |
| GA94 | 2.522 | 2,095.36 | 830.83 | 537.33 | CV2, % | 9.30 |
| GA95 | 1.635 | 1,264.97 | 773.68 | 500.37 | Recovery, % | 104 |
| GA96 | 1.499 | 1,104.59 | 736.88 | 476.57 | n | 5 |
| GA97 | 1.270 | 1,031.42 | 812.14 | 525.24 | Mean, ppm | 506.92 |
| GA98 | 1.902 | 1,513.91 | 795.96 | 514.77 | Std Dev, ppm | 23.22 |
| GA99 | 2.101 | 1,719.76 | 818.54 | 529.38 | CV2, % | 4.58 |
| GA100 | 1,356 | 1,024.56 | 755.57 | 488.66 | Recovery, % | 101 |
* Excluded from statistical analysis
** (@ NTP)
| Notes: | (1) Sampling time taken | = | 60 minutes for first set |
| = | 30 minutes for second set | ||
| = | 120 minutes for third setet | ||
| = | 100 minutes for fourth set | ||
| (2) Sampling rate taken | = | 10 - 20 cc/min | |
High Humidity Test
for
Landauer Passive Monitors
| 60% RH and 25 °C | 90% RH and 25 °C | ||||
| Known | N2O Found | Known N2O Conc. | N2O Found | ||
| ppm** | ppm** | ppm** | ppm** | ||
| 500.00 | 622.50 | 500.00 | 570.63 | ||
| 571.26 | 483.90 | ||||
| 623.51 | 571.69 | ||||
| 633.46 | 589.16 | ||||
| Lost In Analysis | 578.82 | ||||
| 603.23 | 584.14 | ||||
| 570.75 | |||||
| 605.28 | |||||
| n | 5 | n | 8 | ||
| Mean | 610.79 | Mean | 569.30 | ||
| Std Dev | 24.66 | Std Dev | 36.45 | ||
| CV2, % | 4.04 | CV2, % | 6.40 | ||
| Recovery % | 122 | Recovery, % | 114 | ||
| where: | |||||
| Recovery = Mean, ppm / Theoretical, ppm, i.e, for 60% RH Recovery = 611 / 500 = 122 % | |||||
| ** (@ NTP) | |||||
Summary of Comparison of Methods
Active Cartridges vs Passive Monitors
| Active Cartridges | Passive Monitors | |
| Theor. conc., ppm | 10 | 10 |
| # of Samples | 10 | 6 |
| Mean, ppm* | 10.86 | 9.58 |
| Std. Dev, ppm | 0.34 | 2.72 |
| CV2, % | 3.1 | 28.3 |
| Recovery, % | 86.9 | 76.6 |
| Theor. Conc., ppm | 25 | 25 |
| # of Samples | 10 | 6 |
| Mean, ppm* | 26.34 | 23.44 |
| Std Dev, ppm | 0.70 | 2.40 |
| CV2, % | 2.6 | 10.2 |
| Recovery, % | 105 | 93.8 |
| Theor. Conc., ppm | 50 | 50 |
| # of Samples | 10 | 6 |
| Mean, ppm* | 46.21 | 45.60 |
| Std Dev, ppm | 1.15 | 5.47 |
| CV2, % | 2.5 | 12.0 |
| Recovery, % | 92.4 | 91.2 |
| Theor. Conc., ppm | 110 | 110 |
| # of Samples | 10 | 6 |
| Mean, ppm* | 109.52 | 103.89 |
| Std Dev, ppm | 1.77 | 3.54 |
| CV2, % | 1.6 | 3.4 |
| Recovery, % | 99.6 | 94.4 |
| Theor. Conc., ppm | 500 | 500 |
| # of Samples | 9 | 5 |
| Mean, ppm* | 545.24 | 610.79 |
| Std Dev, ppm | 20.81 | 24.66 |
| CV2, % | 3.8 | 4.0 |
| Recovery, % | 109 | 122 |
| Theor. Conc., ppm | 500 | 500 |
| # of Samples | 19 | 8 |
| Mean, ppm | 516.59* | 569.30* |
| Std Dev, ppm | 41.36 | 36.45 |
| CV2, % | 8.0 | 6.4 |
| Recovery, % | 103 | 114 |
| CV2 (pooled), % | 4.9 | 13.5 |
| Avg. Recovery, % | 99.3 | 98.7 |
| * (@NTP) | ||
Note:
- (1) All samples, active cartridges and passive monitors, were
analyzed by the manufacturer, R.S. Landauer, Jr. Co.
(2) The same environmental conditions were used for comparison
(3) The flowrates of 10 to 20 cc/min were used for active samples.
(4) The sampling times were varied from 30 to 120 minutes for active samples and from 120 to 420 minutes for passive samples.
(5) Recovery = Mean, ppm / Theoretical, ppm, e.g., for the sixth set Passive Monitor results above, Recovery = 569/500 = 114%
- Adjust the total flow rate in the dynamic generation system to
determine the theoretical generated N2O
concentration which is based on the flow rate controlled by the mass
flow controller from the N2O gas tank.
For example:
Total flow rate = 20 L/minN2O flow rate = 2.0 cc/min
Therefore, the theoretical N2O concentration in the generation system is equal to 100 ppm.
- Remove each monitor from the package and record the sample number.
- Before exposing to the exposure chamber, attach (or hang) a minimum of six monitors on the Teflon sticker.
- Remove each cap from the head of each monitor by using a "breaking" motion and store the cap by snapping it snugly onto the base of the monitor.
- Open the top of the exposure chamber and put the Teflon sticker with the monitors into the chamber.
- Close and tighten the top by using a metal U-clamp to prevent leakage between the top and the chamber.
- Simultaneously expose the monitors and the active cartridges.
- Remove the active cartridges after 30 or 60 minutes at a flow rate of between 10 and 20 cc/min, depending on the nitrous oxide concentrations.
- At the conclusion of the sampling period, immediately remove the sticker from the chamber and the monitors from the sticker.
- Record the end time and total exposure time.
- Remove each cap from the base of each monitor by using a "breaking" motion and close the monitor by snapping the cap snugly to the head.
- Seal and pack the monitors before sending to the manufacturer for analysis.
FIELD COMPARISON OF NITROUS OXIDE SAMPLING PROCEDURES
Note: This field study was conducted by Ed Zimowski, Senior Industrial Hygienist, OSHA Health Response Team (HRT), Salt Lake City, UT. The study was performed during 1983-84.
BACKGROUND
The adverse health effects of waste anesthetic gases and vapors have been documented for some time. Several epidemiological studies have shown an increased incidence of spontaneous abortions and congenital abnormalities in the children of both female workers and wives of male workers exposed to anesthetic gases. Studies show that exposed personnel have suffered damage to the liver and kidneys and demonstrated symptoms of possible central nervous system effects.
Although OSHA presently does not have a Permissible Exposure Limit for employees exposed to anesthetic gases, NIOSH has recommended a limit in Criteria for a Recommended Standard. . . Occupational Exposure to Waste Anesthetic Gases and Vapors published in March, 1977. NIOSH recommends that when used as the sole anesthetic agent, no worker be exposed to TWA concentrations of nitrous oxide greater than 25 ppm during anesthetic administration. With available technology, exposure levels of 50 ppm and less for nitrous oxide are attainable in dental offices. [After this study was performed the American Conference of Governmental Industrial Hygienists (ACGIH) adopted in 1989 a TWA Threshold Limit Value (TLV) of 50 ppm nitrous oxide.]
SAMPLING PROCEDURES
Until recently, the only procedure available for monitoring employee exposure to nitrous oxide was sampling in gas sampling bags and subsequent infrared (IR) analysis using a direct reading portable infrared analyzer (OSHA has used the MIRAN® IA, 1B, or 103). This normally involved pre- and post-sampling calibration of the instrument in the laboratory and shipment of the equipment to the local area office requesting the equipment. Due to the potential of the instrument losing calibration during shipment and lack of trained field personnel familiar with the equipment, it was often necessary for an HRT member to travel to the local area office to perform training and to assist the compliance officer in the inspection.
Due to the difficulties involved with monitoring employee exposure to nitrous oxide, a field comparison of sampling procedures was performed. The methods chosen for comparison included: (1) gas sampling bag collection and analysis by IR spectrophotometry, (2) Siemens Gammasonics, Inc. nitrous oxide monitor, and (3) R.S. Landauer NITROX® dosimetry system. During the evaluation Siemens withdrew their dosimeter from the market.
SAMPLING PROTOCOL
Area air samples were collected in the operatory rooms of a dental clinic by placing two Landauer dosimeters, one on either side of a piece of Tygon® tubing. The Tygon tube was attached to the vacuum side of a Du Pont P125 sampling pump. The exhaust port of the pump was connected by Tygon tubing to the inlet of an evacuated 20-liter multi-layer sampling bag (Calibrated Instruments, Inc., Ardsley, NY). Area sampling was used because it allowed a wider range of concentrations to be studied. Due to the low sampling rate of the dosimeters, approximately 2 cc/min, starvation of the badges would not occur. Samples were collected for a minimum of 2 hours.
SAMPLE ANALYSIS
The Landauer dosimeters were returned to the manufacturer for analysis because the method of analysis is proprietary. The sampling bags were analyzed on-site using a MIRAN® 103 portable IR with a 4.5 micron nitrous oxide filter. The MIRAN® was pre- and post-calibrated at the OSHA HRT Laboratory in Salt Lake City, UT with a Teflon calibration loop by injecting known amounts of pure nitrous oxide into the cell with a gas-tight syringe. A calibration curve was plotted using the actual concentration of nitrous oxide in the calibration loop as the abscissa and the IR scale readings as the ordinate. Each sampling bag was analyzed by attaching the inlet port to the IR and recording the scale reading. The reading was then converted to ppm at Salt Lake City's altitude (640 mmHg) using the calibration curve. This value was then converted to ppm at 760 mmHg for comparison with the passive dosimeters.
RESULTS
The results of the comparison sampling with the passive dosimeters and the multi-layer sampling bags is shown in Figure 2. Data is shown below. The two dosimeter values represent the duplicate samples collected with each bag sample.
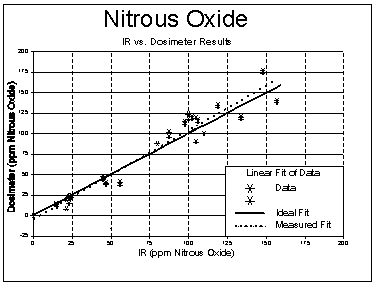
Nitrous Oxide Field Comparison Data (760 mmHg, 25 °C)
| Sampling Bag | Landauer NITROX® Dosimeter | ||
| (ppm N2O) | (ppm N2O) | ||
| 14.6 | 14, | 13 | |
| 15.2 | 11, | 12 | |
| 21.3 | 8, | 19 | |
| 22.8 | 22, | 23 | |
| 23.2 | 15, | 21 | |
| 23.3 | 25, | 23 | |
| 23.8 | 21, | 22 | |
| 24.2 | 22, | 25 | |
| 24.5 | 22, | 22 | |
| 45 | 44, | 48 | |
| 47 | 40, | 38 | |
| 56 | 41, | 37 | |
| 80 | 88, | 88 | |
| 87.6 | 96, | 102 | |
| 97.7 | 115, | 111 | |
| 100 | 124, | 118 | |
| 102 | 118, | 121 | |
| 105 | 119, | 90 | |
| 106 | 117, | 114 | |
| 110 | 100 | ||
| 119 | 132, | 135 | |
| 134 | 118, | 120 | |
| 148 | 174, | 177 | |
| 157 | 140, | 138 | |
CONCLUSIONS
The Landauer NITROX® passive dosimeter provides an acceptable alternative to bag sampling for monitoring employee exposure to nitrous oxide. Linear regression analysis applied to the comparison data using the sampling bag procedure as the reference provided the following information.
| Correlation Coefficient | 0.973 |
| Slope | 1.08 |
| Std. Dev. of the slope | 0.038 |
| Intercept | -3.81 |
| Std. Dev. of the intercept | 3.17 |
| Std. Dev. about the regression line | 12.0 |
The correlation coefficient shows that the range of values studied was adequate to obtain comparison information although it could have been improved by sampling at higher levels. Because of the NIOSH Recommended Exposure Limit, it was necessary to obtain data at the low concentration values. Also, the badges are designed for 40-hour exposure monitoring of hospital and dental employees and therefore the comparative data for short term (2-4 hour) periods was important.
A full laboratory validation was performed in 1985 by the Inorganic Methods Evaluation Branch on the badges and included determination of detection limits, breakthough, accuracy, and stability.
[COMMENT1]Second revised on 08/15/85, okayed by Lee revision2
nitrousoxidepass rev2 11/14/85 from Kenison comments corrected by JCK on
12/19/85 pm