Introduction
The procedure for collection and analysis of air samples for chlorine dioxide (ClO2) is described in OSHA Method No. ID-202 (9.1.). Chlorine dioxide and chlorine (Cl2) are both collected in a midget fritted glass bubbler (MFGB), containing 0.02% potassium iodide (KI) in a weak buffer. These two species are trapped and converted to chlorite (ClO2-) and chloride (Cl-), respectively, based on the following chemical reactions:
These reactions occur in neutral or weakly basic solutions. The collection solution used for this method contains 0.02% KI in 1.5 mM sodium carbonate and 1.5 mM sodium bicarbonate. The collected chlorine dioxide (as ClO2-) and chlorine (as Cl-) are then analyzed by ion chromatography (IC).
This method has been validated for a 120-L, 240-min sample based on a flow rate of 0.5 L/min. The method validation was conducted near the OSHA time weighted average (TWA) permissible exposure limit (PEL) of 0.1 ppm and consisted of the following experiments and summaries:
- 1. An analysis of 18 samples (6 samples at each test level).
2. A sampling and analysis of 18 samples (6 samples at each test level, 50% RH) collected from dynamically generated test atmospheres. Additional samples at other test levels and humidities were also taken.
3. A determination of the sampling media collection efficiency at 0.2 ppm (2 times the TWA PEL).
4. A determination of breakthrough.
5. An evaluation of room temperature storage stability for 12 collected samples.
6. A determination of any significant effects on results when sampling at different humidities.
7. A determination of the qualitative and quantitative detection limits.
8. A determination of sampling efficiency of the collection solution when sampling a mixture of dynamically generated ClO2 and Cl2.
9. Summary.
All theoretical (known) concentrations of generated test atmospheres were determined using the NIOSH chlorophenol red (CPR) method for ClO2 (9.2.). All sampling tests performed were conducted side-by-side with IC and CPR samples being taken and analyzed using the conditions recommended in their methods (9.1., 9.2.). The CPR method was slightly modified for these experiments. The chlorite stock solution was prepared without the addition of acetic anhydride and the solution was standardized using a primary standard instead of molar absorbance as mentioned in the NIOSH method. The unknown potential effect on the IC determinations from having small amounts of acetic anhydride in the standards and not in the samples was one reason for it's exclusion. The chlorite stock solution was standardized using the procedure advocated by the National Council of the Paper Industry for Air and Stream Improvement (NCASI) (9.3.) and the acetic anhydride may also have presented an effect on this titration. This standardization was felt to be more accurate than the NIOSH approach.
All results were calculated from concentration-response curves and statistically examined for outliers. In addition, the analysis (Section 1) and sampling and analysis results (Section 2) were tested for homogeneity of variance. Possible outliers were determined using the Treatment of Outliers test (9.4.). Homogeneity of variance was determined using the Bartlett's test (9.5.). Statistical evaluation was conducted according to Inorganic Methods Evaluation Protocol (9.6.). Overall error (9.6.) was calculated using the equation:
Where i is the respective sample pool being examined.
1. Analysis
- 1.1. Preparation of Known ClO2
Concentrations
Samples were prepared by adding known amounts of sodium chlorite (NaClO2) solution into 25-mL volumetric flasks containing collection solution. Technical-grade NaClO2 was used to prepare the stock solution and was standardized according to the procedure described in the method (9.1.).
1.2. Analysis of Spiked Samples
Analysis was performed using an ion chromatograph equipped with a conductivity detector (9.1.).
1.3. Determination of Analytical Method Recovery (AMR)
Recoveries were compared to the known amounts of chlorite spikes and are presented in Table 1. All results passed the Test for Outliers and the Bartlett's test. The AMR was 97.8% and the analytical precision (CV1 pooled) was 0.024.
2. Sampling and Analysis
- 2.1. Preparation and Collection of Known Generated Samples
- 2.1.1. Dynamic generation system
A diagram of the generation system is shown in Figure 1. The system consists of five essential elements: A flow-temperature-humidity control system (Miller-Nelson Research Inc., Monterey, CA, Model HCS-301) which is used for air flow control and conditioning, a ClO2 or ClO2 + Cl2 mixture vapor generating system, a mixing chamber, and sampling manifold. All generation system fittings and connections were Teflon. A glass mixing chamber was used.
2.1.2. Chlorine dioxide vapor generation system
Chlorine dioxide, a very unstable gas, is extraordinarily reactive and commercially unavailable. Special techniques are required to produce it. For this study, the technique selected involved the passage of a dilute stream of Cl2 vapor through a concentrated aqueous solution of NaClO2, (specifically, 10 g of NaClO2 in 25 mL of deionized water) to produce ClO2 by the reaction:
The Cl2 source was a cylinder containing 530 ppm Cl2 in nitrogen (certified, Airco, Phoenix, AZ). This technique produced a chlorine-free stream of ClO2 vapor. The components exposed to this analyte vapor were composed of glass, Teflon, or other suitably inert materials. The entire system was shielded from light and was operated within the confines of an exhaust hood.
All known (taken) concentrations of ClO2 were determined by the chlorophenol red (CPR) reference method (9.2.). The CPR samples were taken from the generation system side-by-side with all IC samples.
The generator was also designed to produce test atmospheres of Cl2 in air as required during the Cl2 + ClO2 mixture study. A vapor-generation system intended to produce steady-state vapor concentrations of ClO2 (and Cl2) at the appropriate test levels was constructed as shown in Figure 2.
2.1.3. The ClO2 (and Cl2) and diluent air flow rates were adjusted using mass flow controllers. The total flow rate of the system was measured before and after each experiment using a dry test meter.
2.1.4. All samples were taken from the sampling manifold using constant flow pumps. Du Pont Model Alpha-l and -2 pumps were used at sample flow rates of 0.5 L/min for IC and 0.2 L/min for CPR samples, respectively.
2.2. Analysis of Generated Samples
As previously mentioned, side-by-side samples were taken for the IC and CPR methods. Samples taken using the KI/buffer were analyzed by IC (9.1.). Analysis of the CPR samples was performed by colorimetry (9.2.). Table 2 shows the sampling and analysis for 0.5, 1, and 2 times OSHA TWA PEL. Table 3 lists a broad range of concentrations of ClO2 from about 0.3 to 3 times the OSHA TWA PEL. Table 4 shows the comparison of results between the IC and CPR samples taken side-by-side.
The data considered to determine precision and accuracy (Table 2) are for 0.5 to 2 times the PEL only [as stated in NIOSH and OSHA Inorganic Methods statistical protocols (9.5., 9.6.)]. The generated sample (Sampling and Analysis - Table 2) results passed the Bartlett's test. Data not passing the Test for Outliers were omitted from final calculations. For 0.5, 1, and 2 times OSHA TWA PEL (Table 2), the pooled coefficients of variation are:
The average recovery of generated samples was 105%. The bias for the overall method was +0.05, and the OE was ±20%.
For all levels tested (0.3 to 3 times the PEL), as shown in Table 3, the pooled CV was 0.072. The bias was +0.033 and the OE was ±18%. All levels tested, presented also in Table 4, gave pooled CVs of 0.035 and 0.072 for CPR and IC samples, respectively.
3. Collection Efficiency and Breakthrough
- 3.1. Collection Efficiency
Procedure: Six samples, each arranged in a sampling train, were collected at a concentration of 2 times the OSHA PEL for 240 min at 0.5 L/min (50% RH and 25 °C). Each sampling train consisted of two MFGBs connected in series and a sampling pump. The amount of ClO2 vapor collected in each of the two MFGBs was determined for each sampling train. The collection efficiency was calculated by dividing the amount collected in the first MFGB by the total amount of ClO2 collected in the first and second MFGBs.
Results: The results in Table 5a show a collection efficiency of 100%.
3.2. Breakthrough (>5% loss of analyte through the sampling media)
Procedure: The same procedure as the collection efficiency experiment was used with one exception: The concentration was varied to include two tests conducted at 0.33 and 0.67 ppm ClO2. A preliminary test was also performed at 1 L/min and about 0.35 ppm (90-min sampling time). The amount of breakthrough was calculated by dividing the amount collected in the second MFGB by the total amount of ClO2 collected in the first and second MFGBs.
Results: For a concentration of 0.33 ppm ClO2, no breakthrough was found after 240 min. For a concentration of 0.67 ppm, the average breakthrough of ClO2 into a second impinger was 9.1%. Results are shown in Table 5b. The preliminary test indicated about 10% breakthrough was noted at a flow rate of 1 L/min (90-min sampling time, about 0.35 ppm ClO2).
4. Storage Stability
Procedure: A study was conducted to assess the stability of ClO2 in the collecting solution. An evaluation was performed of the room temperature storage stability of 12 samples taken near the OSHA TWA PEL of 0.1 ppm. The first test (6 samples) was conducted at 0.07 ppm. When noting an increase in concentration in these samples after 15 days of storage, a second test was performed (6 samples at 0.13 ppm). All samples were stored under normal laboratory conditions (20 to 25 °C) on a lab bench and were not protected from light. An aliquot from each of the samples was analyzed after various periods of storage.
Results: For the storage stability study conducted at 0.07 ppm ClO2, a 11% increase in recoveries occurred after 15 days of storage and then stayed constant through the 102 day study. The mean of samples analyzed after 102 days was within 15% of the mean of samples analyzed the first day.
Results of the room temperature stability study of samples taken at 0.13 ppm (Table 6) show that samples can be stored at ambient (20 to 25 °C) laboratory conditions. A positive bias was not evident during this 96 day study. The mean of samples analyzed after 96 days was still within ±10% of the mean of samples analyzed after 1 day of storage.
5. Humidity Study
Procedure: A study was conducted to test the effect of different humidities during sample collection. Generation system samples were taken using the procedure described in Section 2. Test atmospheres were generated at 25 °C and at the OSHA PEL. Relative humidities of 26, 50, and 80% were used.
Results: Results are listed in Table 7. An F test was used to determine if any significant effect occurred when sampling at different humidities. As shown, a significant difference is not noted when using the F test. This indicates no significant change in results occurred in the humidity ranges tested.
6. Mixture Study
Procedure: In order to determine if the presence of Cl2 can affect the analysis of ClO2, a mixture of Cl2 and ClO2 at 25 °C and 50% RH was generated, and 12 samples were taken using this and the CPR method (6 side-by-side samples for each method). The system used to generate the mixture is described in Section 2 and illustrated in Figure 2.
Results: The known (taken) concentrations of Cl2 and ClO2 were measured individually prior to the experiment using the IC and CPR methods, respectively. The IC method results for both Cl2 and ClO2 after mixing the two gases are shown in Table 8 (Note: A correction was applied to the results of the CPR method due to the positive interference from Cl2 on the ClO2 analysis - for further information regarding this interference, see reference 9.2.). As shown in Table 8, a decrease in recovery (89.5%) occurred for the collection and IC analysis of ClO2.
7. Detection Limit Study
Procedure: Low concentration samples were prepared by spiking solutions with standardized sodium chlorite. A 50-µL sample injection loop and a detector setting of 1 µS was used for all analyses.
Qualitative and quantitative detection limit:
A modification or derivation of the International Union of Pure and Applied Chemistry (IUPAC) detection limit equation (9.7.) was used in this case. At the sensitivity level tested, blank readings and the standard deviation of the blank were equal to zero. The lack of a blank signal does not satisfy a strict interpretation of the IUPAC detection limit calculations. The detection limits for this method were calculated using a standard below the range of the expected detection limit as a substitute for the blank readings.
Results: The results are shown in Table 9 for qualitative and quantitative detection limits, respectively. The qualitative limit is 0.025 µg/mL as ClO2- (using a 50-µL sample injection loop) at the 99.8% confidence level. The quantitative limit is 0.082 µg/mL as ClO2-. Using a 120-L air volume and a 15-mL sample volume, the qualitative limit is 0.001 ppm and the quantitative limit is 0.004 ppm ClO2.
8. Summary
The validation results indicate the method meets either NIOSH or OSHA criteria for accuracy and precision (9.5., 9.6.). Collection efficiency, breakthrough, and storage stability are adequate; however, breakthrough did occur at approximately seven times the TWA PEL and the storage test at 0.07 ppm revealed an increase in recoveries as the test progressed. The reason for the increase in concentration is unknown. The stock standard should be standardized at least monthly. It was noted during testing that this standard solution decreases in concentration approximately 4% per month.
No significant difference in results was noted when sampling at different humidities. As shown in the mixture study, Cl2 does not interfere with the sampling or ion chromatographic analysis of ClO2 at the concentrations tested. Although a resultant 10% decrease in ClO2 and 7% increase in Cl2 concentrations occurred, this could have been due to the difficulty in generating both gases simultaneously. A mixture of ClO2 and Cl2 can be collected and analyzed together; however, Cl2 measurements are considered for screening purposes only. Further work is necessary to validate the KI/buffer sampling and IC analysis for Cl2.
Detection limits are adequate if samples are taken for 240 min at 0.5 L/min. Although no samples were taken to determine ability for Short-Term Exposure Limit (STEL) monitoring, the method appears capable of STEL determinations if a sampling rate of 0.5 L/min is used for at least 15 min. This sampling strategy gives a detection limit of 0.059 ppm for 15-min samples.
9. References
- 9.1. Occupational Safety and Health Administration Technical
Center: Chlorine Dioxide in Workplace Atmospheres by J.C.
Ku (OSHA-SLTC Method No. ID-202). Salt Lake City, UT. Revised 1991.
9.2. National Institute for Occupational Safety and Health: Methods Development for Sampling and Analysis of Chlorine, Chlorine Dioxide, Bromine, and Iodine - Research Report for Chlorine Dioxide by W.K. Fowler and H.K. Dillon. Birmingham, AL: Southern Research Institute (Contract no. 210-80-0067), 1982.
9.3. National Council of the Paper Industry for Air and Stream Improvement, Inc.: A Laboratory Investigation of an Iodometric Method for Determining Chlorine and Chlorine Dioxide in Pulp and Paper Industry Workplace Atmospheres (Technical Bulletin No. 409). New York: NCASI, September 1983.
9.4. Mandel, J.: Accuracy and Precision, Evaluation and Interpretation of Analytical Results, The Treatment of Outliers. In Treatise On Analytical Chemistry, 2nd edition, edited by I.M. Kolthoff and P.J. Elving. New York: John Wiley and Sons, 1978. pp 282-285.
9.5. National Institute for Occupational Safety and Health: Documentation of the NIOSH Validation Tests by D. Taylor, R. Kupel and J. Bryant (DHEW/NIOSH Pub. No. 77-185). Cincinnati, OH: National Institute for Occupational Safety and Health, 1977. pp. 1-12.
9.6. Occupational Safety and Health Administration Analytical Laboratory: Precision and Accuracy Data Protocol for Laboratory Validations. In OSHA Analytical Methods Manual. Cincinnati, OH: American Conference of Governmental Industrial Hygienists (Pub. No. ISBN: 0-936712-66-X), 1985.
9.7. Long, G.L. and J.D. Winefordner: Limit of Detection -- A Closer Look at the IUPAC Definition. Anal. Chem. 55: 712A-724A (1983).
| (OSHA-PEL) | |||||||
|
| |||||||
| µg | µg | ||||||
| Taken | Found | F/T | n | Mean | Std Dev | CV1 | OEa |
|
| |||||||
| (0.5 X PEL) | |||||||
| 16.56 | 17.21 | 1.039 | |||||
| 16.56 | 16.68 | 1.007 | |||||
| 16.56 | 16.07 | 0.970 | |||||
| 16.56 | 17.14 | 1.035 | |||||
| 16.56 | 16.47 | 0.995 | |||||
| 16.56 | 15.81 | 0.955 | |||||
| 6 | 1.000 | 0.034 | 0.034 | 6.8 | |||
| (1 X PEL) | |||||||
| 33.12 | 31.81 | 0.960 | |||||
| 33.12 | 32.07 | 0.968 | |||||
| 33.12 | 32.07 | 0.968 | |||||
| 33.12 | 32.69 | 0.987 | |||||
| 33.12 | 32.10 | 0.969 | |||||
| 33.12 | 31.48 | 0.950 | |||||
| 6 | 0.967 | 0.012 | 0.012 | 5.8 | |||
| (2 X PEL) | |||||||
| 64.24 | 61.50 | 0.957 | |||||
| 64.24 | 62.00 | 0.965 | |||||
| 64.24 | 62.70 | 0.977 | |||||
| 64.24 | 63.81 | 0.993 | |||||
| 64.24 | 62.19 | 0.968 | |||||
| 64.24 | 60.13 | 0.936 | |||||
| 6 | 0.966 | 0.019 | 0.020 | 7.4 | |||
| Analytical Method Recovery (AMR) = 0.978 | |||||||
|
| |||||||
| F/T | = | Found/Taken |
| OEa | = | ± Overall Error (Analytical) |
| Bias | = | -0.022 |
| CV1 (Pooled) | = | 0.024 |
| Overall Error (Analytical) | = | ±7.0% |
| (OSHA-PEL) | |||||||
|
| |||||||
| ppm | ppm | ||||||
| Taken | Found | F/T | n | Mean | Std Dev | CV2 | OEs |
|
| |||||||
| (0.5 X PEL) | |||||||
| 0.058 | 0.076 | 1.310 | |||||
| 0.058 | 0.059 | 1.017 | |||||
| 0.058 | 0.058 | 1.000 | |||||
| 0.058 | 0.067 | 1.155 | |||||
| 0.058 | 0.065 | 1.121 | |||||
| 0.058 | 0.071 | 1.224 | |||||
| 6 | 1.138 | 0.119 | 0.105 | 34.8 | |||
| (1 X PEL) | |||||||
| 0.107 | 0.112 | 1.047 | |||||
| 0.107 | 0.106 | 0.991 | |||||
| 0.107 | 0.108 | 1.009 | |||||
| 0.107 | 0.110 | 1.028 | |||||
| 0.107 | 0.124 | 1.159 | |||||
| 0.107 | 0.116 | 1.084 | |||||
| 6 | 1.053 | 0.061 | 0.058 | 16.9 | |||
| (2 X PEL) | |||||||
| 0.202 | 0.192 | 0.950 | |||||
| 0.202 | 0.200 | 0.990 | |||||
| 0.202 | 0.200 | 0.990 | |||||
| 0.202 | 0.189 | 0.936 | |||||
| 0.202 | 0.205 | 1.015 | |||||
| 0.202 | 0.177 | 0.876 | |||||
| 6 | 0.960 | 0.050 | 0.052 | 14.5 | |||
|
| |||||||
| F/T | = | Found/Taken |
| OEs | = | ± Overall Error (Sampling and Analysis) |
| Bias | = | +0.050 |
| CV2 (Pooled) | = | 0.075 |
| CVT (Pooled) | = | 0.076 |
| Overall Error (Total) | = | ±20.1% |
|
| ||||||||
| Test Level | Air Vol | Found | Taken | -----------Statistical Analysis------------------ | ||||
| (L) | ppm | ppm | n | Mean | Std Dev | CV2 | Recovery (%) | |
|
| ||||||||
| 0.3 X PEL | 120 | 0.030 | 0.028 | |||||
| (25 °C & | 116 | 0.026 | 0.028 | |||||
| 50% RH) | 118 | 0.029 | 0.028 | |||||
| 120 | 0.024 | 0.028 | ||||||
| 91 | 0.037 | 0.028 | ||||||
| 107 | 0.023 | 0.028 | ||||||
| 6 | 0.028 | 0.005 | 0.18 | 101 | ||||
| 0.6 X PEL | 118 | 0.076 | 0.058 | |||||
| (25 °C & | 114 | 0.059 | 0.058 | |||||
| 28% RH) | 117 | 0.058 | 0.058 | |||||
| 119 | 0.067 | 0.058 | ||||||
| 120 | 0.065 | 0.058 | ||||||
| 106 | 0.071 | 0.058 | ||||||
| 6 | 0.066 | 0.007 | 0.105 | 114 | ||||
| 0.7 X PEL | 118 | 0.069 | 0.071 | |||||
| (25 °C & | 115 | 0.066 | 0.071 | |||||
| 80% RH) | 117 | 0.070 | 0.071 | |||||
| 119 | 0.065 | 0.071 | ||||||
| 120 | 0.068 | 0.071 | ||||||
| 119 | 0.094* | 0.071 | ||||||
| 5 | 0.068 | 0.002 | 0.031 | 95.2 | ||||
| 0.7 X PEL | 116 | 0.074 | 0.072 | |||||
| (25 °C & | 114 | 0.077 | 0.072 | |||||
| 50% RH) | 117 | 0.081 | 0.072 | |||||
| 117 | 0.077 | 0.072 | ||||||
| 119 | 0.078 | 0.072 | ||||||
| 129 | 0.088 | 0.072 | ||||||
| 6 | 0.079 | 0.005 | 0.062 | 110 | ||||
| 1 X PEL | 120 | 0.112 | 0.107 | |||||
| (25 °C & | 116 | 0.106 | 0.107 | |||||
| 26% RH) | 118 | 0.108 | 0.107 | |||||
| 120 | 0.110 | 0.107 | ||||||
| 122 | 0.124 | 0.107 | ||||||
| 124 | 0.116 | 0.107 | ||||||
| 6 | 0.113 | 0.007 | 0.058 | 105 | ||||
| 1 X PEL | 118 | 0.094 | 0.087 | |||||
| (25 °C & | 115 | 0.094 | 0.087 | |||||
| 80% RH) | 117 | 0.093 | 0.087 | |||||
| 119 | 0.090 | 0.087 | ||||||
| 120 | 0.092 | 0.087 | ||||||
| 119 | 0.129* | 0.087 | ||||||
| 5 | 0.093 | 0.002 | 0.018 | 106 | ||||
| 1.3 X PEL | 116 | 0.133 | 0.130 | |||||
| (25 °C & | 116 | 0.133 | 0.130 | |||||
| 50% RH) | 112 | 0.128 | 0.130 | |||||
| 116 | 0.128 | 0.130 | ||||||
| 117 | 0.126 | 0.130 | ||||||
| 119 | 0.115 | 0.130 | ||||||
| 6 | 0.126 | 0.006 | 0.047 | 97.1 | ||||
| 2 X PEL | 119 | 0.241 | 0.212 | |||||
| (25 °C & | 116 | 0.241 | 0.212 | |||||
| 28% RH) | 118 | 0.240 | 0.212 | |||||
| 119 | 0.244 | 0.212 | ||||||
| 121 | 0.243 | 0.212 | ||||||
| 122 | 0.234 | 0.212 | ||||||
| 6 | 0.241 | 0.004 | 0.015 | 113 | ||||
| 2 X PEL | 90 | 0.192 | 0.202 | |||||
| (25 °C & | 115 | 0.200 | 0.202 | |||||
| 50% RH) | 119 | 0.200 | 0.202 | |||||
| 120 | 0.189 | 0.202 | ||||||
| 120 | 0.205 | 0.202 | ||||||
| 116 | 0.177 | 0.202 | ||||||
| 6 | 0.194 | 0.010 | 0.052 | 96.0 | ||||
| 2 X PEL | 118 | 0.174 | 180 | |||||
| (25 °C & | 115 | 0.176 | 0.180 | |||||
| 80% RH) | 118 | 0.184 | 0.180 | |||||
| 120 | 0.176 | 0.180 | ||||||
| 121 | 0.183 | 0.180 | ||||||
| 120 | 0.259* | 0.180 | ||||||
| 5 | 0.179 | 0.005 | 0.026 | 99.2 | ||||
| 3 X PEL | 119 | 0.319 | 0.330 | |||||
| (25 °C & | 117 | 0.315 | 0.330 | |||||
| 50% RH) | 119 | 0.317 | 0.330 | |||||
| 119 | 0.324 | 0.330 | ||||||
| 122 | 0.314 | 0.330 | ||||||
| 116 | 0.236* | 0.330 | ||||||
| 5 | 0.318 | 0.004 | 0.012 | 96.3 | ||||
| 3 X PEL | 118 | 0.312 | 0.288 | |||||
| (25 °C & | 114 | 0.310 | 0.288 | |||||
| 80% RH) | 118 | 0.299 | 0.288 | |||||
| 119 | 0.309 | 0.288 | ||||||
| 120 | 0.312 | 0.288 | ||||||
| 122 | 0.326 | 0.288 | ||||||
| 6 | 0.311 | 0.009 | 0.028 | 108 | ||||
|
| ||||||||
* Outlier - not used in statistical analysis
| All concentration levels | ||
| CV2 (pooled) | = | 0.072 |
| Bias | = | +0.033 |
| Overall Error | = | ±18% |
|
| |||
| (a) 30% RH & 25 °C | |||
|
| |||
| CPR | IC | IC/CPR | |
| n | 6 | 6 | |
| Mean (ppm) | 0.058 | 0.066 | 1.14 |
| Std Dev (ppm) | 0.004 | 0.007 | |
| CV2 | 0.069 | 0.105 | |
| n | 6 | 6 | |
| Mean (ppm) | 0.107 | 0.113 | 1.05 |
| Std Dev (ppm) | 0.007 | 0.007 | |
| CV2 | 0.067 | 0.058 | |
| n | 6 | 6 | |
| Mean (ppm) | 0.212 | 0.241 | 1.13 |
| Std Dev (ppm) | 0.006 | 0.004 | |
| CV2 | 0.029 | 0.015 | |
|
| |||
| (b)50% RH & 25 °C | |||
|
| |||
| n | 6 | 6 | |
| Mean (ppm) | 0.028 | 0.028 | 1.01 |
| Std Dev (ppm) | 0.002 | 0.005 | |
| CV2 | 0.079 | 0.18 | |
| n | 6 | 6 | |
| Mean (ppm) | 0.072 | 0.079 | 1.10 |
| Std Dev (ppm) | 0.002 | 0.005 | |
| CV2 | 0.029 | 0.062 | |
| n | 6 | 6 | |
| Mean (ppm) | 0.130 | 0.126 | 0.969 |
| Std Dev (ppm) | 0.010 | 0.006 | |
| CV2 | 0.077 | 0.047 | |
| n | 6 | 6 | |
| Mean (ppm) | 0.202 | 0.194 | 0.960 |
| Std Dev (ppm) | 0.019 | 0.010 | |
| CV2 | 0.095 | 0.052 | |
| n | 6 | 6 | |
| Mean (ppm) | 0.330 | 0.318 | 0.963 |
| Std Dev (ppm) | 0.013 | 0.004 | |
| CV2 | 0.039 | 0.012 | |
|
| |||
| (c) 80% RH & 25 °C | |||
|
| |||
| n | 6 | 5 | |
| Mean (ppm) | 0.071 | 0.068 | 0.958 |
| Std Dev (ppm) | 0.002 | 0.002 | |
| CV2 | 0.031 | 0.031 | |
| n | 6 | 5 | |
| Mean (ppm) | 0.087 | 0.093 | 1.06 |
| Std Dev (ppm) | 0.003 | 0.002 | |
| CV2 | 0.033 | 0.018 | |
| n | 6 | 5 | |
| Mean (ppm) | 0.180 | 0.179 | 0.992 |
| Std Dev (ppm) | 0.006 | 0.005 | |
| CV2 | 0.034 | 0.026 | |
| n | 6 | 6 | |
| Mean (ppm) | 0.288 | 0.311 | 1.08 |
| Std Dev (ppm) | 0.026 | 0.009 | |
| CV2 | 0.089 | 0.028 | |
|
| |||
| All Levels | |||
|
|
|||
| CV2 (pooled) | 0.035 | 0.072 | |
|
| |||
| ---------ppm Chlorine Dioxide--------- | |||
| Sample No. | First Bubbler | Second Bubbler | % Collection Efficiency |
|
| |||
| 1 | 0.192 | ND | 100 |
| 2 | 0.200 | ND | 100 |
| 3 | 0.200 | ND | 100 |
| 4 | 0.189 | ND | 100 |
| 5 | 0.205 | ND | 100 |
| 6 | 0.177 | ND | 100 |
|
| |||
| Note: | (1) | Sampled at 0.5 L/min for 240 min |
| (2) | Sampling solution = 25 mL | |
| (3) | ND = None detectable, < 0.02 ppm ClO2 |
|
| |||||||||||||||
| --------ppm ClO2 Found---------- | |||||||||||||||
| Sample No. | 1st Bubbler | 2nd Bubbler | % Breakthrough | ||||||||||||
|
| |||||||||||||||
| 1 | 0.319 | ND | 0 | ||||||||||||
| 2 | 0.315 | ND | 0 | ||||||||||||
| 3 | 0.317 | ND | 0 | ||||||||||||
| 4 | 0.324 | ND | 0 | ||||||||||||
| 5 | 0.314 | ND | 0 | ||||||||||||
| no breakthrough | |||||||||||||||
|
| |||||||||||||||
| 6 | 0.686 | 0.076 | 9.97 | ||||||||||||
| 7 | 0.682 | 0.066 | 8.82 | ||||||||||||
| 8 | 0.666 | 0.081 | 10.84 | ||||||||||||
| 9 | 0.680 | 0.062 | 8.36 | ||||||||||||
| 10 | 0.680 | 0.062 | 8.36 | ||||||||||||
| 11 | 0.633 | 0.057 | 8.26 | ||||||||||||
| 9.1% breakthrough | |||||||||||||||
| |||||||||||||||
|
| |||||||||||||||
| Note: | (1) | Sampled at 0.5 L/min for 240 min |
| (2) | Sampling solution = 25 mL | |
| (3) | ND = None detectable, < 0.02 ppm C102 |
|
| ||||||||
| Test Level | Air Vol | Found | Taken | ------------Statistical Analysis----------------- | ||||
| 0.13 ppm ClO2 | (L) | ppm | ppm | n | Mean | Std Dev | CV | Recovery (%) |
|
| ||||||||
| Day 1 | 116 | 0.133 | 0.130 | |||||
| 112 | 0.128 | 0.130 | ||||||
| 116 | 0.128 | 0.130 | ||||||
| 117 | 0.126 | 0.130 | ||||||
| 119 | 0.115 | 0.130 | ||||||
| 104 | 0.127 | 0.130 | ||||||
| 6 | 0.126 | 0.006 | 0.047 | 97.1 | ||||
| Day 5 | 116 | 0.125 | 0.130 | |||||
| 112 | 0.122 | 0.130 | ||||||
| 116 | 0.117 | 0.130 | ||||||
| 117 | 0.123 | 0.130 | ||||||
| 119 | 0.125 | 0.130 | ||||||
| 104 | 0.118 | 0.130 | ||||||
| 6 | 0.122 | 0.003 | 0.028 | 93.6 | ||||
| Day 15 | 116 | 0.133 | 0.130 | |||||
| 112 | 0.129 | 0.130 | ||||||
| 116 | 0.127 | 0.130 | ||||||
| 117 | 0.125 | 0.130 | ||||||
| 119 | 0.131 | 0.130 | ||||||
| 104 | 0.157* | 0.130 | ||||||
| 5 | 0.129 | 0.003 | 0.025 | 99.2 | ||||
| Day 30 | 116 | 0.126 | 0.130 | |||||
| 112 | 0.130 | 0.130 | ||||||
| 116 | 0.130 | 0.130 | ||||||
| 117 | 0.128 | 0.130 | ||||||
| 119 | 0.125 | 0.130 | ||||||
| 104 | 0.161* | 0.130 | ||||||
| 5 | 0.128 | 0.002 | 0.018 | 98.3 | ||||
| Day 48 | 116 | 0.131 | 0.130 | |||||
| 112 | 0.131 | 0.130 | ||||||
| 116 | 0.127 | 0.130 | ||||||
| 117 | 0.128 | 0.130 | ||||||
| 119 | 0.127 | 0.130 | ||||||
| 104 | 0.164* | 0.130 | ||||||
| 5 | 0.129 | 0.002 | 0.016 | 99.1 | ||||
| Day 96 | 116 | 0.137 | 0.130 | |||||
| 112 | 0.132 | 0.130 | ||||||
| 116 | 0.128 | 0.130 | ||||||
| 117 | 0.133 | 0.130 | ||||||
| 119 | LIA | 0.130 | ||||||
| 104 | 0.161* | 0.130 | ||||||
| 4 | 0.133 | 0.004 | 0.028 | 102 | ||||
|
| ||||||||
| LIA = Lost in Analysis | ||||||||
| * Outlier - not used in statistical analysis | ||||||||
|
| |||||
| % RH | 26 | 50 | 80 | ||
|
| |||||
| ppm ClO2 Taken | 0.107 | 0.130 | 0.087 | ||
| ppm ClO2 Found | 0.112 | 0.133 | 0.094 | ||
| 0.106 | 0.128 | 0.094 | |||
| 0.108 | 0.128 | 0.093 | |||
| 0.110 | 0.126 | 0.090 | |||
| 0.124 | 0.115 | 0.092 | |||
| 0.116 | 0.127 | 0.129* | |||
| n | = | 6 | 6 | 5 | |
| Mean (ppm) | = | 0.113 | 0.126 | 0.093 | |
| Std Dev (ppm) | = | 0.007 | 0.006 | 0.002 | |
| CV | = | 0.058 | 0.047 | 0.018 | |
| Ave Recovery | = | 105% | 97.1% | 106% | |
| * Excluded from statistical analysis as an outlier. | |||||
|
| |||||
| At the 95% confidence level: Fcrit = 3.68 Fcalc = 3.39 (2, 15 degrees of freedom) Fcrit > Fcalc; therefore, a significant difference in results was not noted across the humidity levels tested. | |||||
|
| |||||
|
| |||||
| ---------Chlorine---------- | Chlorine Dioxide | ||||
| Taken* | Found** | Taken*** | Found** | ||
| Air Vol, L | ppm | ppm | ppm | ppm | |
|
| |||||
| 29 | 1.56 | 1.62 | 0.556 | 0.488 | |
| 27 | 1.56 | 1.72 | 0.556 | 0.497 | |
| 29 | 1.56 | 1.70 | 0.556 | 0.481 | |
| 29 | 1.56 | 1.71 | 0.556 | 0.514 | |
| 28 | 1.56 | 1.66 | 0.556 | 0.499 | |
| 26 | 1.56 | 1.60 | 0.556 | 0.506 | |
| n | = | 6 | 6 | ||
| Mean | = | 1.67 | 0.498 | ||
| Std Dev | = | 0.05 | 0.012 | ||
| CV | = | 0.03 | 0.024 | ||
| Recovery | = | 107% | 89.5% | ||
|
| |||||
| * | MFGB samples containing KI/buffer. These samples were collected from the chlorine atmosphere immediately before mixing. They were analyzed by IC. | ||
| ** | MFGB samples containing KI/buffer. These samples were collected after mixing the chlorine and chlorine dioxide. They were analyzed by IC. | ||
| *** | CPR samples. These samples collected after mixing and then analyzed using NIOSH CPR method. This result is corrected for the influence of chlorine. | ||
| Note: | Samples were also taken using the CPR method immediately before mixing. The results of all CPR samples indicated: | ||
| 1) | CPR samples taken of only the generated chlorine gave 0.133 ppm as chlorine dioxide. | ||
| 2) | CPR samples taken of the mixture gave 0.689 ppm as chlorine dioxide. | ||
| 3) | Therefore, the correction for the chlorine dioxide Taken ppm was: | ||
| 0.689 - 0.133 = 0.556 ppm | |||
|
| ||||
| ---------Chlorine Dioxide (as ClO2) Level--------- | ||||
| 0.10 µg/mL | 0.20 µg/mL | 0.50 µg/mL | ||
| Sample No. | PA | PA | PA | |
|
| ||||
| 1 | 0.966 | 2.806 | 14.05 | |
| 2 | 1.187 | 4.338 | 14.87 | |
| 3 | 0.890 | 3.391 | 15.56 | |
| 4 | 1.210 | 3.986 | 14.27 | |
| 5 | 1.619 | 3.064 | 15.54 | |
| 6 | 1.520 | 3.368 | 15.79 | |
| n | = | 6 | 6 | 6 |
| Mean | = | 1.232 | 3.492 | 15.01 |
| Std dev | = | 0.291 | 0.573 | 0.732 |
| CV | = | 0.236 | 0.164 | 0.049 |
| PA = Integrated Peak Area (ClO2-)/100,000 | ||||
|
| ||||
| Using the equation: | Cld = k(sd)/m |
| Where: | ||
| Cld | = | the smallest reliable detectable concentration an analytical instrument can determine at a given confidence level. |
| k | = | 3 (Qualitative Detection Limit, 99.86% Confidence) |
| = | 10 (Quantitative Detection Limit, 99.99% Confidence) | |
| sd | = | standard deviation of the 0.1 µg/mL standard readings. |
| m | = | analytical sensitivity or slope as calculated by linear regression. |
| Cld | = | 3(0.291)/35.35 = 0.025 µg/mL ClO2- for the qualitative limit. |
| Cld | = | 10(0.291)/35.35 = 0.082 µg/mL ClO2- for the quantitative limit. |
Qualitative detection limit = 0.38 µg
ClO2- (15-mL sample volume) or
0.001 ppm ClO2
Quantitative detection limit = 1.23 µg
ClO2- (15-mL sample volume) or
0.004 ppm ClO2
The system shown below provided a means for generating dynamic test atmospheres. The system consists of four essential elements: a flow-temperature-humidity control system, a chlorine dioxide (and chlorine) vapor generating system (see Figure 2), a mixing chamber, and an active sampling manifold.
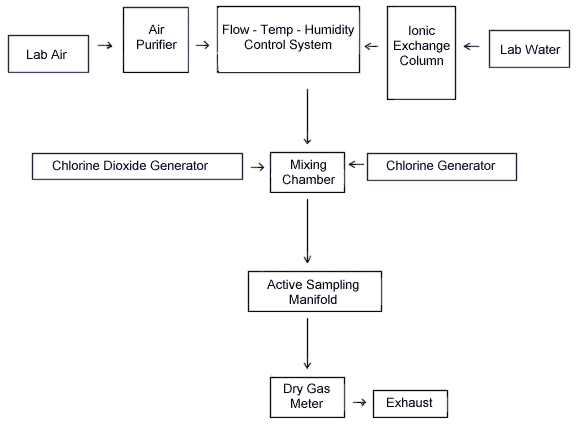
The equipment shown below provided a means for dynamic generation of chlorine dioxide and chlorine test atmospheres.
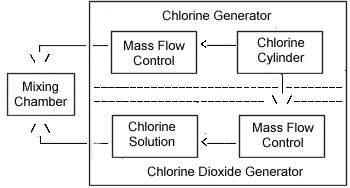
* 10 g NaClO2 in 25 mL deionized water