3M FORMALDEHYDE MONITOR (MODEL 3721)
| Classification: | Product Evaluation (PE-10) (Backup Data Report for OSHA Method ID-205) |
| Date: | July, 1989 |
| Authors: | James Ku(1), Ed Zimowski(2), CIH |
descriptive use only and do not constitute endorsements by USDOL-OSHA.
OSHA Technical Center
Salt Lake City, Utah
Introduction
A field and laboratory evaluation of the 3M model 3721 formaldehyde
monitor was conducted by the OSHA Health Response Team
Previous studies (9.1.-9.3.) of formaldehyde passive sampling and
analysis were conducted with the model 3751 monitor. The OSHA permissible
exposure limit (PEL) was a
| Previous Standards | Adopted Standards |
| 3 ppm TWA | 1 ppm TWA |
| 5 ppm Ceiling | 2 ppm STEL* |
| 10 ppm Peak | ---------- |
| *STEL = Short-Term Exposure Limit | |
These changes signaled a need to re-examine the performance of this
monitor. This
Field Study: Samples were collected by the
Lab Study: This portion of the product evaluation was
conducted at the OSHA Laboratory to determine if the
1. History
The simplicity and freedom of the 3M formaldehyde passive monitor (model 3751) showed promise when first offered in 1981 as an industrial hygiene sampling alternative for formaldehyde (9.8.); however, subsequent independent studies indicated analyte loss when sampling at low humidities (9.1., 9.3.). Consequently, the model 3751 monitor was removed from the market by 3M in April, 1984. The model 3721 3M monitor, capable of sample humidification, was introduced in 1985 as a replacement. The changes instituted by 3M and incorporated into the model 3721 are:
- A water saturated pad in the bottom section of the monitor has been added for sample humidification.
- Each monitor is now packaged in a sealed metal container. Previously, the model 3751 monitor was enclosed in a resealable plastic bag.
- The calculated sampling rate has been changed from 0.0659 liters per minute (L/min) to 0.0614 L/min*.
| The sampling rate of 0.0614 L/min is in agreement with a previous OSHA Laboratory study (9.2.). |
With the exception of the moisturizing pad, the model 3721 appears physically identical to the model 3751 monitor.
The model 3751 monitor has been extensively evaluated by independent
laboratories
Sampling and analytical procedures are identical for either model monitor; however, result calculations are different since slightly different sampling rates are used.
2. Principle of the 3M Monitor
The 3M formaldehyde monitor is a diffusion-type air monitoring
assembly. The monitor is worn in the breathing zone of personnel in order
to evaluate potential exposure to formaldehyde vapors contained in the
atmosphere. The formaldehyde vapor passes through a diffusion barrier and
is adsorbed on
The formaldehyde collected by the monitor is laboratory analyzed by
desorbing the
3. Evaluation Criteria
Field Study: The monitor lot numbers used during the
field study were unavailable. All monitors were tested before their
expiration dates. The field experiments involved taking
Personnel and area samples were taken in various occupational
environments where exposures to formaldehyde were near 1 ppm as a TWA.
Exposure levels were estimated using
Results for the 3M monitor were compared to the treated
Lab Study: The laboratory experiments were mainly concerned with:
- Comparing the performance of the monitors near the 1 ppm PEL with a reference method
- Assessing performance in a low humidity environment (<50% RH)
- Taking STEL measurements (approximately 2 ppm for 15 min)
The 3M sampling and analytical method (9.5., 9.6.) was compared
Monitors obtained from 3M for the lab portion of the evaluation were
from lots 6125 002 (exp. date 10/20/87) and 8092 001 (exp. date 10/6/89).
All monitors were exposed and analyzed in the lab before their expiration
dates. The former lot was tested about 1 week before the expiration date.
(Note: A previous study (9.3.) of the 3751 monitor uncovered a
Monitor performance was determined using formaldehyde test atmosphere
concentrations of approximately 0.4, 1, 1.5, 3, and 5 × PEL and calculated
using the OSHA Laboratory Inorganic Method Statistical Evaluation Protocol
(9.12.). Testing was mainly conducted for
In addition, a separate experiment was conducted at the OSHA Laboratory
comparing the Field Study reference method with the Lab Study reference
method. This experiment was conducted by taking
All bubbler, tube, and monitor samples collected for the Field or Lab Studies were analyzed at the OSHA Lab. All monitor samples were analyzed according to the analytical procedure provided by 3M (9.6.).
4. Formaldehyde Test Atmospheres
Field Study: The formaldehyde concentration of samples
taken in different wood products industries was dependent on the type of
adhesive or resin mixture used, amount of production, and press
temperature. The source of exposure was mainly from either
urea-formaldehyde (UF) or
Lab Study: The apparatus used to generate dynamic test atmospheres of formaldehyde for lab tests is shown as a block diagram in Figure 1. The system consisted of five essential elements:
- A vapor generating chamber (permeation oven)
- A humidity control system
- A mixing manifold
- An aluminum passive monitor exposure channel
- A sampling manifold for active samplers
The formaldehyde was generated using permeation tubes containing reagent grade paraformaldehyde. The permeation device used for generation of formaldehyde vapor was a model 450 Dynacalibrator (VICI/Metronics, Santa Clara, CA). The permeation device chamber was maintained at a constant temperature of 110 °C. Concentrations of formaldehyde in the generation system were preliminarily determined by the weight change of the permeation tube. Weight changes are shown below:
| Time Elapsed (min) |
Weight Loss (µg) | Permeation Rate (µg/min) |
| 3,975 | 215,100 | 54.11 |
| 8,605 | 468,900 | 54.49 |
| Average 54.30 ± 0.27 µg/min | ||
Purified air for contaminant dilution was prepared by flowing
compressed air through a particulate filter, a silica gel bed, and a
charcoal bed. Humidified air for dilution was produced by flowing the
purified air into a flow, temperature, and humidity control system
A controlled formaldehyde concentration was produced by taking filtered
room air and passing it over the permeation tube, and then mixing with the
The contaminant-air mixture then entered a Teflon sampling manifold and eventually a calibrated dry test meter (Singer Co., model no. DTM 115) for flow rate measurement. The flow rate of the formaldehyde vapor to the mixing chamber was approximately 0.17 L/min. Depending on the final concentration desired, the flow rate range for dilution air was 5 to 25 L/min.
An aluminum exposure channel containing six openings, obtained from 3M and also used in a previous OSHA study (9.2.), was connected to an arm of the sampling manifold. This channel, as shown in Figure 2, was used to test 5 monitors per experiment. The sixth port was sealed using an empty monitor.
Monitor results were compared to the reference method sample results to determine recovery ratios. The bias and overall error in a previous study of the reference method (9.10., 9.11.) were +2.9% and ±19% respectively. Theoretical results using the weight change of permeation tubes were not used in the statistical analysis due to a small leak that developed in the generation system during low concentration testing (tests done at <1 × PEL) . This leakage altered the actual generated concentration and apparently was a result of the increased dilution volume necessary for low concentration testing.
5. Sampling
Field Study: The field sampling technique provided by 3M
(9.5.) was used for taking monitor samples. Full shift samples were taken.
Tubes containing
Lab Study: The monitor and reference samples were exposed
using the following
- Adjust diluent flow rates in the dynamic generation system to determine an approximate generated HCHO concentration. Adjust the system to deliver either 30, 50, or 80% RH.
- Record the sample number and the exposure start time on the back of each monitor.
- Before exposing, wrap parafilm around the edge of each monitor to prevent leakage between each monitor and the exposure channel.
- For the reference samples, calibrate personal sampling pumps to approximately 0.5 L/min sampling rates. Add approximately 15 mL of 10% methanol (MeOH) to midget fritted glass bubblers (MFGB).
- Place the monitors in the exposure channel and attach MFGBs to the sampling manifold. Simultaneously expose the bubblers and the monitors.
- At the conclusion of the sampling period, immediately remove and discard the white plastic film and purple retaining ring from the top of the monitor. Also remove the bottom portion which contains the metal clip.
- Firmly snap the closure caps onto the monitor faces.
- Place the MFGB solutions into 25-mL volumetric flasks and dilute to the mark with 10% MeOH. Post-calibrate the sampling pumps.
- Record the exposure end time on the back of the monitor.
- Enclose the monitor in the original aluminum container until ready for analysis.
This procedure (a to j) was repeated for each
As previously mentioned, reference MFGB samples were taken according the procedures outlined in references 9.10 and 9.11. The MFGB sampling times for the TWA determinations varied between 30 and 240 min. Six MFGB and five monitor samples were tested at each concentration/humidity level. All monitor samples were exposed for 240 min except for the STEL experiment which took 15 min.
The exposure channel inlet was connected to the manifold and the outlet was connected to a Du Pont P4000 sampling pump whose sampling rate was set at 3 to 5 L/min. This flow range produced face velocities inside the channel of 9.2 to 15.3 m/min (30 to 50 ft/min). As shown in Figure 2, the monitor samples are placed in series in the channel such that the formaldehyde mixture contacts the first and then subsequent monitors. If the flow rate through the channel is less than 6 L/min, a "starvation effect" is produced from one monitor to another. The flow rates in this 3M sampling chamber were less than 6 L/min and all results were corrected for this "starvation" using the following equation (9.9.):
| Cn = | Co × [FR - (n - 1) SR]
FR |
Where:
| Cn | = | HCHO theoretical concn (in ppm) as sampled by the nth sampler |
| Co | = | HCHO (in ppm) in the generation system |
| FR | = | Air flow rate in the sampling chamber |
| SR | = | Diffusional sampling rate for the contaminant (0.0614 L/min) |
| n | = | Placement in the channel; the monitor first exposed to the formaldehyde mixture would have a value of n = 1, and n = 5 for the last monitor exposed in the channel. |
6. Analysis
Field and Lab Studies - 3M Monitors: The analytical procedure used was the NIOSH P&CAM 125 procedure for analysis of formaldehyde (9.13.) which has been slightly modified by 3M. Modifications were apparently instituted to alter the analytical working range and minimize interferences. These modifications include:
- A smaller total sample volume (3 mL) and greater sample/aliquot
ratio (3/2) are used.
- A change from a ratio of 4.0: 0.1: 6.0 mL (NIOSH method) to 2.0:
1.0: 5.0 mL (3M monitor method) for the analytical reagents listed
below.
sample: 1% chromotropic acid: concentrated sulfuric acid
The formaldehyde adsorbed on the bisulfite-impregnated adsorbent was analyzed according to the procedure described below (9.6.):
- The formaldehyde-bisulfite adduct was desorbed by adding 3 mL of
deionized water (DI H2O) through the center
port of the elutriation cap (outer cap) using a pipette. The port was
re-sealed. - The sample was allowed to sit for 30 min with occasional gentle
agitation. A
2-mL aliquot of the solution was then transferred into a20-mL screw-cap glass vial. - A purple color was produced in this aliquot after addition of 1 mL of 1% chromotropic acid solution along with slow and careful addition of 5 mL concentrated sulfuric acid. This mixture was agitated and then allowed to cool to room temperature.
- The absorbance was measured with a UV-VIS Model Spectronic 100
single beam spectrophotometer (Bausch & Lomb, Rochester, NY) or a
double beam CARY 219
UV-VIS spectrophotometer (Varian Associates, Palo Alto, CA) at 580 nm using a1-cm cell.
Field Study - Solid sorbent samples: The resulting derivative
[formaldehyde plus
Lab Study (MFGB samples): The 10% MeOH solutions were buffered, hydrazine was added, and the resulting hydrazone compound was analyzed by square wave polarography. This technique was used instead of the differential pulse polarographic technique mentioned in reference 9.10. and 9.11. because it offered a decrease in analysis time with comparable results.
7. Results
The weight of formaldehyde collected by each monitor was calculated a
polynomial regression
|
(1) |
Where:
| C | = | Average formaldehyde concentration (ppm) | |
| W | = | Weight (from curve, as µg) | |
| MV | = | Molar volume (at 25 °C and 760 mmHg, MV = 24.45 L/mole) | |
| MW | = | Molecular weight of formaldehyde (30 g/mole) | |
| K | = | Monitor sampling rate for formaldehyde (0.0614 L/min) | |
| RC | = | Recovery coefficient for formaldehyde | |
| t | = | Length of sampling period (min) |
Since RC = 1.00 (9.2., 9.6.), equation (1) was simplified to:
|
(2) |
Sample results for solid sorbent and bubbler samples were calculated according to their respective methods (9.7., 9.10.) The results of the experiments can be found in these Tables:
| Experiment |
Table | ||
| 3M monitors vs. Treated XAD-2 (Field Study) | 1 (also Figure 3) | ||
| 3M monitors - 240 min samples (Lab Study) | 2 | ||
| 3M monitors - STEL experiment (Lab Study) | 3 | ||
| 3M monitors - Summary (Lab Study) | 4 (also Figure 4) | ||
| Treated XAD-2 vs. MFGB Samplers | 5 | ||
Field Study: Shown below are the results from linear regression plotting of the data shown in Table 1 and Figure 3:
| Correlation coefficient | (r) = | 0.976 |
| Intercept | (a) = | -0.0059 |
| Slope | (b) = | 0.952 |
| Standard deviation of slope | (Sb) = | 0.037 |
When comparing two different methods by linear regression, ideal
agreement between methods is displayed if the correlation coefficient and
the slope are equal to a value of 1. As shown in Figure 3, the dashed line
corresponding to ideal agreement does not differ greatly from the
regression line calculated from field results. When considering the slope
and the standard deviation of the slope at 95% confidence (0.952 ± 2 ×
0.037), the slope does not significantly differ from a value of 1. The
correlation coefficient is also close to 1. Therefore, the 3M and
Average blank values for monitors analyzed during the Field Study were approximately 1 µg.
Lab Study: Table 2 contains individual monitor results for the three RH levels and five concentrations tested. A positive bias is noted when comparing monitors with MFGBs. As previously mentioned, generation system leakage at low concentrations prevented theoretical results from being used and therefore, monitor results were only compared with MFGB results. Theoretical results at generated concentrations greater than the PEL were available and a comparison with monitor results gave an acceptable overall error (9.12.) of ±21.7%. When comparing the monitor and MFGB recoveries using theoretical values in this concentration range, the MFGB average recovery was 8.7% less than the monitors.
Table 3 contains results for the five monitors taken at the STEL. The STEL measurements indicate somewhat variable results are obtained when sampling for a short duration.
Table 4 contains a summary of Table 2 data and F test results. As shown in Table 4, the monitor displayed a total recovery ratio of 1.064 and thus, a positive overall bias of 6.4%. Figure 4 graphically displays the comparison of recovery ratios for the monitor and MFGB samples. Each point on the graph is an average of five or six sample recovery ratios at a given concentration and RH. In this Figure, ideal agreement between the two methods is shown by the dashed line. Bias is displayed when the solid line (slope of actual measurements) moves towards either axis. Positive bias is shown by the solid line leaning toward the 3M axis.
An F test was used to detect any significant variability in results across the three humidity levels tested. The F test monitor results in Table 4 show a significant difference does exist at the 99% confidence level. An examination of recovery ratios at the different RH levels (Table 2) indicates an enhancement at 80% RH. Recovery ratios were approximately 10% higher at 80% RH. A shift in performance due to humidity was not noted for the reference method.
Blank values for monitors analyzed during the Lab Study ranged from 0.91 to 3.39 µg with a CV of 0.55. If the 3.39 µg measurement is omitted, the blank values are much closer with a range of 0.91 to 1.5 µg and a CV of 0.21.
The comparison of the treated XAD-2 sampling device with MFGBs showed
agreement between the two methods (Table 5). A slight positive bias of the
8. Summary
The 3M model 3721 monitor and selected reference methods gave similar
formaldehyde results in field and lab experiments when samples were taken
for at least 240 min. The field study indicates the monitor results were
not significantly different from the treated
Short-term sampling with the monitor may give highly variable results. This variability could be due to insufficient equilibration time or to the decreased amount of analyte taken. The time necessary to passivate the surfaces of the monitor and to achieve a homogeneous sampling rate may be longer than 15 min. Any analytical method has difficulty when measuring amounts of analyte near the method's detection limit. The total amount of formaldehyde generated at the STEL was approximately 2 µg. This amount is near the analytical detection limit described by 3M (9.9.). Even if this amount is detectable using the procedure described, the blank values noted in the Lab Study were approximately 1 to 3 µg. If the blank value is not consistent, the measurement of any sample near the blank value can become erratic and inaccurate.
As previously mentioned, during the Field Study the concentration of formaldehyde usually fluctuated over the sampling period. The variability in concentration did not appear to affect the ability of the monitor to sample. These results agree with a previous study (9.2) performed using fluctuating formaldehyde concentrations under laboratory conditions.
Concentrations and humidities used to test the monitor were in the
range normally expected in workplace situations. Overall results indicate
the monitor gave similar results to the 10% methanol MFGBs and treated
9. References
- 9.1. National Council of the Paper Industry for Air and Stream
Improvement Inc.: A Laboratory Evaluation on the Performance of
Passive Diffusion Badge Monitors and Detector Tubes for Determination
of Formaldehyde. (Technical Bulletin No. 451), NY: NCASI, 1985.
9.2. Occupational Safety and Health Administration Analytical
Laboratory: Evaluation of 3M Formaldehyde Monitors (Model
3751) by J.C. Ku (USDOL/OSHA-SLCAL Product Evaluation no.
9.3. Kennedy, E.R. and R.D. Hull: Evaluation of the Du Pont
9.4. "Formaldehyde," Code of Federal Regulations Title 29, Pt.
1910, Section 1048. 1988. pp.
9.5. Occupational Health and Safety Products Division/3M: 3M Formaldehyde Monitor #3721 Instructions for Use. St. Paul, MN: 3M Company. No publication date given.
9.6. Occupational Health and Safety Products Laboratory: Organic Vapor Method no. 4D. St. Paul, MN: 3M Company, May, 1985.
9.7. Occupational Safety and Health Administration Analytical
Laboratory: OSHA Analytical Methods Manual
9.8. Rodriguez, S.T., P.B. Olson, and V.R. Lund: "Colorimetric Analysis of Formaldehyde Collected on a Diffusional Monitor." Paper presented at Amer. Ind. Hyg. Assoc. Conference, Portland, OR, May 1981.
9.9. Occupational Health and Safety Products Division/3M: 3M Brand Formaldehyde Monitor #3750/3751. St. Paul, MN: 3M Company, Internal document - No publication date given.
9.10. Occupational Safety and Health Administration Analytical
Laboratory: OSHA Analytical Methods Manual
9.11. Septon, J.C. and J.C. Ku: Workplace Air Sampling and Polarographic Determination of Formaldehyde. Amer. Ind. Hyg. Assoc. J. 43:845-852 (1982).
9.12. Occupational Safety and Health Administration Analytical
Laboratory: Precision and Accuracy Data Protocol for Laboratory
Validations. In The OSHA Laboratory Methods Manual. Cincinnati,
OH: American Conference of Governmental Industrial Hygienists (Pub No.
ISBN:
9.13. National Institute of Occupational Safety and Health:
NIOSH Manual of Analytical Methods, 1st ed. (P&CAM 125)
edited by D. Taylor (DHHS/NIOSH Pub.
| Company |
Type |
Operation |
XAD-2 (ppm) |
3M (ppm) | |||
| E E E E F F A A B D A A E A A A C A H H A C H C E G B F F F F F F B D |
Ar Ar Ar Ar P P P P P Ar P P Ar P P P P P Ar Ar P Ar Ar P Ar Ar P Ar Ar Ar Ar Ar Ar P Ar |
Press Operator Press Operator Press Unloader Press Unloader Press Operator Press Operator Patcher Core Feeder On Walkway Above Conveyer (mat former) Strip Stacker Production Saw Patcher Big Dry Chain Grader Top of Press Dry End Fork Lift Core Feeder Little Dry Chain Grader Utility Big Dryer Feeder Style Grain Topcoat Operator Topcoat Operator Small Dryer Feeder Adjacent to Cooling Wheel Laminating Line - Topcoat Operator Cleanup Top of Press - Glue Mixing Press Operator On Walkway above Conveyer to Hot Press Top of Press Top of Press Top of Press Top of Press Top of Press Top of Press Above Conveyer Top of Press |
0.12 0.12 0.12 0.15 0.11 0.13 0.21 0.26 0.27 0.21 0.28 0.22 0.26 0.26 0.30 0.26 0.38 0.18 0.43 0.52 0.25 0.60 0.43 0.52 0.69 0.78 1.12 0.90 0.99 1.14 0.17 1.28 1.26 2.12 2.42 |
0.082 0.084 0.092 0.10 0.14 0.13 0.20 0.21 0.22 0.22 0.23 0.24 0.24 0.27 0.28 0.30 0.30 0.28 0.35 0.35 0.36 0.44 0.44 0.57 0.60 0.81 0.93 0.97 1.35 1.16 1.06 1.06 1.26 1.90 2.42 | |||
| Company |
B = Particleboard plant (highly automated) using
C = Particleboard plant using UF resin
D = Particleboard plant using UF resin
E = Hardwood plywood plant using UF resin
F = Hardwood plywood plant using UF resin
G = Medium density fiberboard plant using UF resin
H = Hardwood paneling plant using topcoat of UF resin
Ar = Area Sample
P = Personal Sample
|
| |||
| HCHO Concn* (ppm) |
HCHO Found (ppm) |
Statistical Analysis | |
|
| |||
| 0.247 | 0.253 | n | = 5 |
| 0.270 | Mean (ppm) | = 0.242 | |
| 0.241 | Std Dev (ppm) | = 0.021 | |
| 0.221 | CV | = 0.086 | |
| 0.223 | Recovery Ratio | = 0.978 | |
| 1.07 | 0.852 | n | = 5 |
| 1.01 | Mean (ppm) | = 0.959 | |
| 0.887 | Std Dev (ppm) | = 0.092 | |
| 0.968 | CV | = 0.096 | |
| 1.08 | Recovery Ratio | = 0.896 | |
| 1.61 | 1.63 | n | = 5 |
| 1.53 | Mean (ppm) | = 1.61 | |
| 1.70 | Std Dev (ppm) | = 0.062 | |
| 1.60 | CV | = 0.039 | |
| 1.59 | Recovery Ratio | = 1.000 | |
| 2.87 | 3.45 | n | = 5 |
| 3.34 | Mean (ppm) | = 3.27 | |
| 3.13 | Std Dev (ppm) | = 0.12 | |
| 3.23 | CV | = 0.038 | |
| 3.22 | Recovery Ratio | = 1.139 | |
| 4.48 | 5.01 | n | = 5 |
| 4.64 | Mean (ppm) | = 5.31 | |
| 5.46 | Std Dev (ppm) | = 0.48 | |
| 5.64 | CV | = 0.090 | |
| 5.81 | Recovery Ratio | = 1.185 | |
| All concentration levels (30%
RH) Recovery Ratio = 1.040 CV(pooled) = 0.075 |
|||
|
| |||
| * Values from average recovery of six MFGB samples analyzed by polarography | |||
| Precision and Accuracy for 3M Formaldehyde Monitors (Model 3721) (b) 50% RH & 25 °C | |||
|
| |||
| HCHO Concn* (ppm) |
HCHO Found (ppm) |
Statistical Analysis | |
|
| |||
| 0.414 | 0.438 | n | = 5 |
| 0.418 | Mean (ppm) | = 0.418 | |
| 0.436 | Std Dev (ppm) | = 0.020 | |
| 0.391 | CV | = 0.047 | |
| 0.407 | Recovery Ratio | = 1.01 | |
| 1.15 | 1.13 | n | = 5 |
| 1.15 | Mean (ppm) | = 1.10 | |
| 1.09 | Std Dev (ppm) | = 0.038 | |
| 1.06 | CV | = 0.035 | |
| 1.08 | Recovery Ratio | = 0.958 | |
| 1.41 | 1.47 | n | = 5 |
| 1.58 | Mean (ppm) | = 1.52 | |
| 1.57 | Std Dev (ppm) | = 0.063 | |
| 1.55 | CV | = 0.041 | |
| 1.44 | Recovery Ratio | = 1.078 | |
| 2.88 | 3.03 | n | = 5 |
| 3.44 | Mean (ppm) | = 2.78 | |
| 2.72 | Std Dev (ppm) | = 0.47 | |
| 2.40 | CV | = 0.169 | |
| 2.30 | Recovery Ratio | = 0.965 | |
| 4.91 | 5.34 | n | = 5 |
| 5.62 | Mean (ppm) | = 5.30 | |
| 5.22 | Std Dev (ppm) | = 0.19 | |
| 5.13 | CV | = 0.036 | |
| 5.21 | Recovery Ratio | = 1.079 | |
| All concentration levels (50%
RH) Recovery Ratio = 1.018 CV(pooled) = 0.084 | |||
|
| |||
| * Values from average recovery of six MFGB samples analyzed by polarography | |||
| Precision and Accuracy for 3M Formaldehyde Monitors (Model 3721) (c) 80% RH & 25 °C | |||
|
| |||
| HCHO Concn* (ppm) |
HCHO Found (ppm) |
Statistical Analysis | |
|
| |||
| 0.460 | 0.533 | n | = 5 |
| 0.568 | Mean (ppm) | = 0.502 | |
| 0.474 | Std Dev (ppm) | = 0.048 | |
| 0.448 | CV | = 0.096 | |
| 0.485 | Recovery Ratio | = 1.090 | |
| 0.910 | 1.04 | n | = 3 |
| 0.975 | Mean (ppm) | = 0.991 | |
| 0.202** | Std Dev (ppm) | = 0.043 | |
| 0.959 | CV | = 0.043 | |
| 0.728** | Recovery Ratio | = 1.089 | |
| 1.47 | 1.69 | n | = 5 |
| 1.77 | Mean (ppm) | = 1.79 | |
| 1.84 | Std Dev (ppm) | = 0.072 | |
| 1.88 | CV | = 0.040 | |
| 1.79 | Recovery Ratio | = 1.218 | |
| 2.91 | 2.83 | n | = 5 |
| 3.37 | Mean (ppm) | = 3.20 | |
| 3.07 | Std Dev (ppm) | = 0.26 | |
| 3.26 | CV | = 0.080 | |
| 3.48 | Recovery Ratio | = 1.100 | |
| 4.62 | 5.28 | n | = 5 |
| 5.71 | Mean (ppm) | = 5.44 | |
| 5.55 | Std Dev (ppm) | = 0.18 | |
| 5.36 | CV | = 0.033 | |
| 5.32 | Recovery Ratio | = 1.177 | |
| All concentration levels (80%
RH) Recovery Ratio = 1.139 CV(pooled) = 0.065 | |||
|
| |||
| * Values from average recovery of six MFGB samples analyzed by polarography | |||
| ** Not included in statistical analysis - considered outliers | |||
|
| |||
| HCHO Concn* (ppm) |
HCHO Found (ppm) |
Statistical Analysis | |
|
| |||
| 2.22 | 3.02 | n | = 5 |
| 1.68 | Mean (ppm) | = 1.91 | |
| 0.975 | Std Dev (ppm) | = 0.737 | |
| 2.03 | CV | = 0.385 | |
| 1.87 | Recovery Ratio | = 0.86 | |
|
| |||
| * Values from average recovery of six MFGB samples analyzed by polarography | |||
|
| |||
| Level | CVT | Recovery Ratio | |
|
| |||
| Below 1 × PEL | 0.079 | 1.026 | |
| 1 × PEL | 0.069 | 0.982 | |
| 1.5 × PEL | 0.040 | 1.099 | |
| 3 × PEL | 0.11 | 1.068 | |
| 5 × PEL | 0.059 | 1.147 | |
|
| |||
|
| ||||
| Recovery Ratio(total) = 1.064 | CV(pooled) = 0.075 | |||
| At the 99% confidence level: | ||||
| Fcrit (3M) | = 4.92 | Fcalc (3M) | = 9.0 | (2, 70 degrees of freedom) |
| Fcrit (MFGB) | = 5.06 | Fcalc (MFGB) | = 0.51 | (2, 50 degrees of freedom*) |
| For the 3M monitors: Fcrit < Fcalc; therefore, a significant difference in results exists across the three RH levels tested. | ||||
| For the MFGB: Fcrit > Fcalc; therefore, a significant difference in results was not noted across the three humidity levels tested. | ||||
|
| ||||
| * Only results > 1 ppm were used. | ||||
|
| ||||
| Sample No. | XAD-2 ppm |
10% MeOH ppm |
XAD-2/10% MeOH | |
|
| ||||
| 1 | 0.870 | 0.849 | ||
| 2 | 0.853 | 0.835 | ||
| 3 | 0.885 | 0.838 | ||
| 4 | 0.862 | 0.814 | ||
| 5 | 0.827 | |||
| n | = 4 | 5 | ||
| Mean | = 0.868 | 0.833 | 1.04 | |
| Std Dev | = 0.014 | 0.013 | ||
| CV | = 0.016 | 0.016 | ||
|
| ||||
| (b) 80% RH and 25 °C | ||||
|
| ||||
| Sample No. | XAD-2 ppm |
10% MeOH ppm |
XAD-2/10% MeOH | |
|
| ||||
| 1 | 1.03 | 0.891 | ||
| 2 | 0.941 | 0.890 | ||
| 3 | 1.01 | 0.917 | ||
| 4 | 1.01 | 0.934 | ||
| 5 | 0.916 | |||
| n | = 4 | 5 | ||
| Mean | = 0.998 | 0.910 | 1.10 | |
| Std Dev | = 0.039 | 0.019 | ||
| CV | = 0.039 | 0.021 | ||
|
| ||||
Block Diagram of the Major components in a Dynamic
Generation System 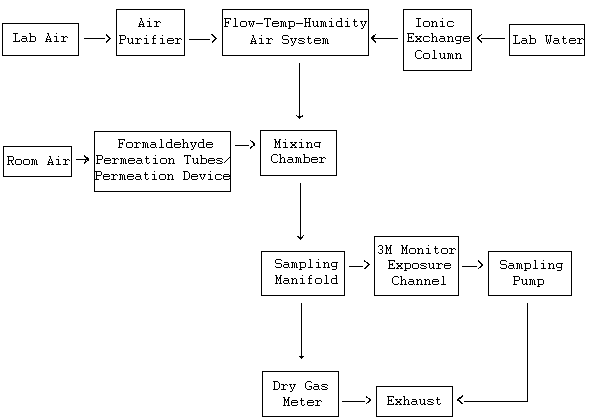
Figure 1
3M Monitor Aluminum Exposure Channel 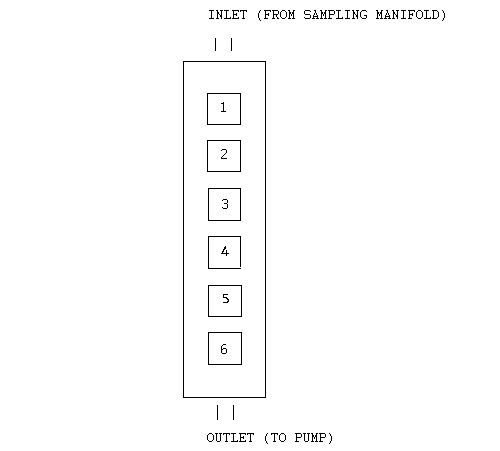
| Note: | The square sampling ports are shown to illustrate sample
placement. The Ports are round on the actual channel. |
Figure 2
Field Study 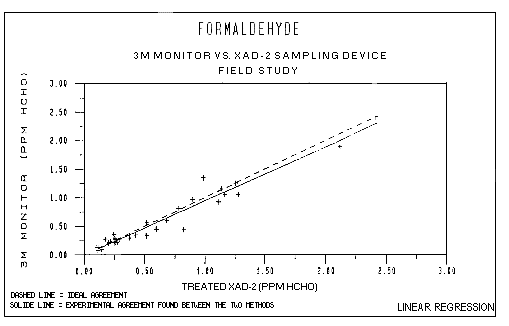
Figure 3
Lab Study 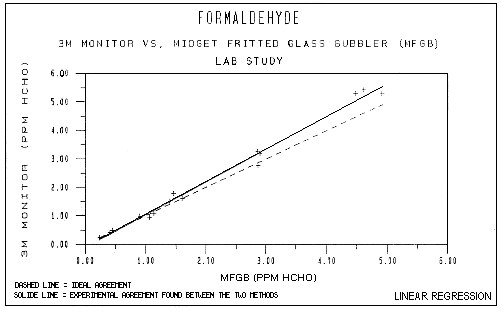
Figure 4
Footnote (2) Senior Industrial Hygienist, Health Response Team, OSHA Technical Center.