| Method no.: | 05 |
| Matrix: | Air |
| Target concentration: | 10 ppm (49 mg/m3) |
| Procedure: | Collection on charcoal adsorbent, desorption with carbon disulfide, analysis by gas chromatography using a flame ionization detector. |
| Detection limit based on recommended air volume: |
0.11 ppm |
| Recommended air volume and sampling rate: |
10 L at 0.2 L/min |
| Standard error of estimate at the target concentration: (Section 4.3.) |
8.5% |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: May 1979 | Chemist: Duane Lee |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
In 1946, the American Conference of Government Industrial Hygienist (ACGIH) published a list of Maximum Allowable Concentrations (MAC) which gave a value of 100 ppm for chloroform. This was changed to a Threshold Limit Value (TLV) and reduced to a time-weighted average of 50 ppm in 1959 (Ref. 5.1.). OSHA has adopted a ceiling value of 50 ppm for chloroform in workplaces (Ref. 5.2.). In March 1976, NIOSH issued a "Current Intelligence Bulletin" on chloroform. The recommendation was made that no worker be exposed to chloroform in excess of 10 ppm determined as a time-weighted average exposure (Ref. 5.3.). For this reason, the analytical and sampling procedures must be evaluated to insure an adequate method exists for lower levels. In FY 1978, the OSHA Analytical Laboratory analyzed 72 samples for chloroform.
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
Animal studies have been conducted on the toxic effects of chloroform. Exposure to chloroform by ingestion, inhalation, and intravenous administration has produced fatty degeneration and necrosis of the liver, and kidney impairment. Inhalation experiments have shown depression of the nervous system, dilation of pupils of the eyes, reduced reaction to light, and reduced intraocular pressure. Studies also indicate carcinogenic effects of chloroform. In rats, it produced epithelial tumors of the kidney. In mice, it produced hepatocellular carcinomas (Ref. 5.3.).
In humans, chloroform was used as an anesthetic. Death from its use as an anesthetic has resulted from liver damage and from cardiac arrest (Ref. 5.4.). Also, an effect from acute exposure is depression of the central nervous system (Ref. 5.5.). From occupational exposure, studies have found episodes of lassitude, dry mouth, depression, irritability and painful urination (Ref 5.3.). There is no evidence of any association of cancer with chloroform exposure.
1.1.3. Worker exposure
NIOSH estimates that 40,000 persons are exposed occupationally to chloroform. The majority of these workers handle small amounts of chloroform (Ref. 5.3.).
1.1.4. Use and operations where exposure occurs
Chloroform was discovered almost simultaneously by Liebig and Soubeirain in 1831 (Ref. 5.6.). Initially, it was used for an anesthetic until the toxic effects were noticed, which reduced its use. Presently, most of the chloroform produced is used in the production of fluorocarbons for refrigerants and fire fighting aids. It is also used as a solvent for extraction of vitamins and purification of antibiotics. During 1974, 302 million pounds of chloroform were produced in the United States (Ref. 5.3.).
Industries where chloroform is used include those producing biological products, pharmaceutical preparations, paint and allied products, paper milling, petroleum refining and metal industries, and surgical supplies, as well as hospitals (Ref. 5.3.).
1.1.5. Physical properties (Refs. 5.1. and 5.5.)
| molecular weight: | 119.39 |
| specific gravity: | 1.49845 at 15/4°C |
| melting point: | -63.5°C |
| boiling point: | 61.26°C |
| vapor pressure: | 200 mm Hg (25°C) |
| vapor density: | 4.1 at 25°C (air=1) |
| color: | colorless liquid |
| chemical formula: | CHCl3 |
| other names: | trichloromethane; trichloroform; methenyl trichloride; formyl trichloride; methyl trichloride |
1.2. Detection limit, precision, sensitivity and working range
- 1.2.1. The detection limit for the analytical procedure is 5.3
ng per injection with a coefficient of variation 0.08 at this level
(Section 4.1.). The detection limit was determined using 1-µL
injections.
1.2.2. The pooled coefficient of variation of the analytical procedure over the range of 0.213 to 1.068 mg per sample is 0.029 (Section 4.2.). This represents an air concentration range of 4.4 to 21.9 ppm based on the recommended sampling and analytical procedures.
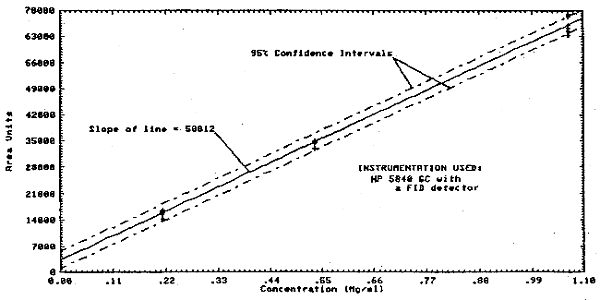
1.2.3. The sensitivity of the analytical procedure over a concentration range representing 0.5 to 2 times the PEL or target concentration based on the recommended air volume is 58812 area units per mg/mL. The sensitivity is determined by the slope of the calibration curve (Section 4.2.). The sensitivity will vary somewhat with the particular instrumentation used in the analysis.
1.2.4. The lower limit of the estimated working range, assuming adequate desorption efficiency is 0.11 ppm. The upper limit of the working range is dependent on the capacity of the collection medium.
1.3. Accuracy
- 1.3.1. The overall procedure must provide results that are
within 25% or better at the 95% confidence interval.
1.3.2. The recovery of analyte from the collection medium after storage must be 75% or greater.
1.3.3. The overall procedure has met the above validation criteria (Section 4.3.).
1.4. Advantages
- 1.4.1. The sampling procedure is convenient.
1.4.2. The analytical procedure is quick, sensitive, and reproducible.
1.4.3. Reanalysis of the samples is possible.
1.5. Disadvantages
If other compounds are present, the GC run time must be lengthened so the late eluting peaks will not interfere with the next sample.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. An approved and calibrated personal sampling pump whose
flow can be determined with ±5% at the recommended flow.
2.1.2. Charcoal tubes: Glass tube, with both ends heat-sealed,
7.0-cm × 6-mm o.d. ×
2.2. Reagents
None required
2.3. Sampling technique
- 2.3.1. Immediately before sampling, break the ends of the
charcoal tube. The size of the holes in the broken ends of the tubes
must be at least one half the i.d. of the tube. All tubes must be
from the same lot.
2.3.2. Connect the charcoal tube to the sampling pump with flexible tubing. The short section of the charcoal tube is used as a backup and should be positioned nearer the sampling pump.
2.3.3. The tube should be placed in a vertical position during sampling to minimize channeling.
2.3.4. Air being sampled should not pass through any hose or tubing before entering the charcoal tube.
2.3.5. Seal the charcoal tube with plastic caps immediately after the sampling.
2.3.6. With each batch of samples, submit at least one blank tube from the same lot used for samples. This tube should be subjected to exactly the same handling as the samples (break, seal, transport) except that no air is drawn through it.
2.3.7. Transport the samples (and corresponding paperwork) to the lab for analysis.
2.3.8. If bulk samples are submitted for analysis, they should be transported in glass containers with Teflon-lined caps. These samples must not be put in the same container used for the charcoal tubes.
2.4. Breakthrough
A sample was taken on the primary portion of a charcoal tube (Lot 107) from an atmosphere containing 20 ppm chloroform and an average relative humidity of 79% at 0.203 L/min. The breakthrough volume was 38.8 L.
2.5. Desorption efficiency
The desorption efficiency, from liquid injections on charcoal tubes (Lot 107), averages 95.9% for 0.24 to 1.1 mg per tube, which is 4.8 to 21.8 ppm for a 10-L air volume (Section 4.4.).
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 10 L.
2.6.2. The recommended sampling rate is 0.2 L/min.
2.7. Interferences (sampling)
- 2.7.1. At the present time, it is unknown if any compound would
severely interfere with the collection of chloroform on charcoal. In
general, the presence of other solvents will decrease the
breakthrough volume for a particular solvent.
2.7.2. Any compound which is suspected of interfering in the collection or analysis should be listed on the sampling data sheet.
2.8. Safety precautions
- 2.8.1. Safety glasses should be worn when breaking the ends of
the tubes.
2.8.2. The broken ends of the tubes should be protected to avoid injury to the person being sampled.
2.8.3. When working in environments containing flammable vapors, do not provide any spark source from equipment used or pumps.
2.8.4. Observe all safety practices for working in hazardous areas.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A gas chromatograph equipped with a flame ionization
detector.
3.1.2. A number of GC columns are available and adequate to separate chloroform from carbon disulfide and the internal standard, which was pentane in this study. The column used for this study was a 10-ft × 1/8-in. stainless steel 0.1% SP-1000 coated on 80/100 mesh Carbopack C.
3.1.3. An electronic integrator or other suitable method of measuring peak area.
3.1.4. Two-milliliter vials with Teflon-lined caps.
3.1.5. Microliter syringes, 10-µL for preparing standards, 1-µL for sample injections.
3.1.6. Pipets for diluting standards. A 1-mL for dispensing
solvent for desorption, or a
3.1.7. Volumetric flasks, convenient sizes for preparing standards.
3.2. Reagents
- 3.2.1. Carbon disulfide, chromatographic grade.
3.2.2. Chloroform, reagent grade.
3.2.3. Pentane, reagent grade, was used as an internal standard.
3.2.4. Helium, hydrogen, and air; purified GC grade.
3.2.5. Desorbing reagent, 1 µL of pentane per milliliter of CS2.
3.3. Standard preparation.
- 3.3.1. Standards are prepared by diluting pure chloroform with
the desorbing reagent.
3.3.2. Nine microliters of chloroform per 25 mL of desorbing reagent is equivalent to 10.9 ppm for a 10-L air sample desorbed with 1 mL of desorbing reagent.
3.4. Sample preparation
- 3.4.1. The front and back sections of each sample are
transferred to separate 2-mL vials.
3.4.2. Each section is desorbed with 1.0 mL of desorbing reagent.
3.4.3. The vials are sealed immediately and allowed to desorb for 30 min with intermediate shaking.
3.5. Analysis
- 3.5.1. GC conditions
| helium (carrier gas) flow rate: | 21.5 mL/min |
| injector temperature: | 200°C |
| detector temperature: | 260°C |
| column temperature: | 100°C |
| detector: | flame ionization |
| hydrogen flow rate: | 65 mL/min |
| air flow rate: | 240 mL/min |
| injection size: | 1 µL |
3.5.2. Chromatogram
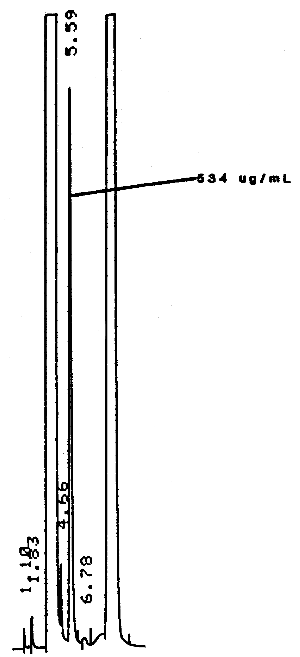
A typical chromatogram of chloroform under the GC conditions used in the analysis is shown in Figure 3.5.2.
3.5.3. Peak areas are measured by an electronic integrator or other suitable means.
3.5.4. An internal standard procedure is used. The integrator is calibrated to report results in ppm for a 10-L air sample after correction for desorption effieiency.
3.6. Interferences (analytical)
- 3.6.1. Any compound having the same general retention time of
chloroform or the internal standard used is an interference.
Possible interferences are listed on the sample data sheets. GC
parameters should be chosen so these interferences will pose no
problems.
3.6.2. GC parameters may be changed to circumvent most interferences.
3.6.3. Retention time on a single column is not considered proof of chemical identity. Samples should be confirmed by GC/MS or other suitable means.
3.7. Calculations
Usually the integrator is programmed to report results in ppm (corrected for desorption efficiency) for a 10-L air sample. The following calculation is used:
| ppm = (A)/(0.1)(B) | where | A | = | ppm on report |
| B | = | air volume (L) | ||
3.8. Safety precautions
- 3.8.1. All work using solvents (preparation of standards,
desorption of samples, etc.) should be done in a hood.
3.8.2. Avoid any skin contact with all of the solvents.
3.8.3. Safety glasses should be worn throughout the procedure.
4. Backup Data
- 4.1. Detection limit
The standard used contained 0.0036 µL chloroform per milliliter of desorbing reagent or 5.34 µg/mL. Peak heights were used since the integrator was not reproducible on areas at this low concentration.
Detection Limit Data
|
| ||||
| injection | peak height (mm) | statistics | ||
|
| ||||
| 1 | 20 | |||
| 2 | 19 | |
= | 20 |
| 3 | 21 | SD | = | 1.69 |
| 4 | 20 | CV | = | 0.08 |
| 5 | 22 | |||
| 6 | 22 | |||
| 7 | 17 | |||
| 8 | 19 | |||
|
| ||||
Detection limit is 5.34 ng per injection (5.34 ng/µL × 1 µL). This is equivalent to 0.109 ppm for a 10-L air sample desorbed with 1.0 mL of desorbing reagent.
4.2. Instrument response and analytical precision
The analytical precision was determined from multiple analyses of three analytical standards whose respective concentrations were about 0.5, 1, and 2 times the target concentration. The area responses from these multiple analyses (and from which the data below were calculated) are plotted in Figure 4.2. and show a linear relationship with concentration.
Instrument Response and Precision Data
|
| |||
| × target conc. | 0.5× | 1× | 2× |
|
| |||
| µg/sample | 274.597 | 583.908 | 1100.31 |
| found | 283.940 | 564.595 | 1107.76 |
| 292.710 | 603.008 | 1122.40 | |
| 256.623 | 607.400 | 1111.86 | |
| 285.008 | 593.089 | 1120.44 | |
| 287.557 | 598.172 | 1124.18 | |
| 283.314 | |||
| 282.109 | |||
| 280.7198 | 591.6953 | 1114.4917 | |
| SD | 10.9899 | 15.5793 | 9.4356 |
| CV | 0.0391 | 0.0263 | 0.00847 |
|
| |||
4.3. Storage data
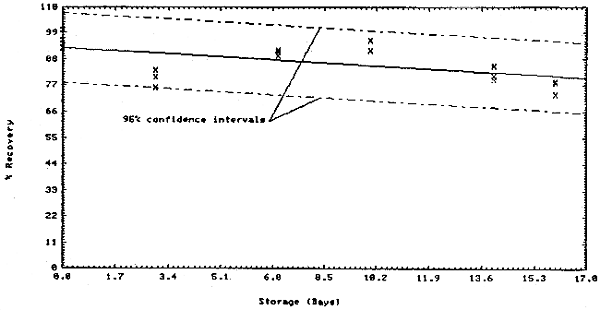
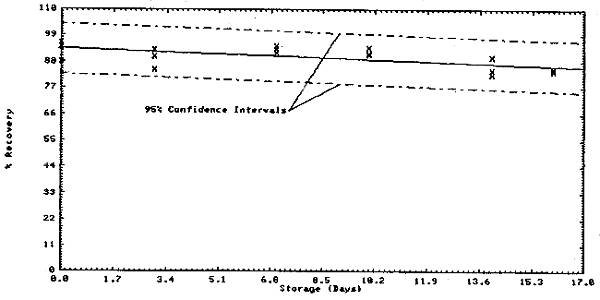
Thirty-six samples were collected on charcoal tubes (SKC Lot 107) from a dynamic test atmosphere containing 10 ppm chloroform and 80% relative humidity. The recommended sampling conditions were used. Six samples were analyzed immediately. The remainder were divided into two equal groups, one stored at room temperature (21 to 26°C) and the other under refrigeration (-1 to 0°C). Three samples from each group were analyzed at 3 or 4 day intervals over 16 days.
Storage Tests
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 | 94.2 | 96.0 | 88.2 | 92.9 | 96.2 | 100.3 | |
| 3 | 85.0 | 93.1 | 90.2 | 76.1 | 80.6 | 83.3 | |
| 7 | 93.1 | 91.7 | 94.6 | 89.6 | 91.0 | 91.7 | |
| 10 | 91.1 | 91.5 | 94.0 | 91.7 | 95.9 | 91.8 | |
| 14 | 82.8 | 84.4 | 89.9 | 79.6 | 85.2 | 81.0 | |
| 16 | 84.0 | 85.1 | 84.0 | 73.1 | 77.9 | 78.8 | |
|
| |||||||
The above data is plotted in Figures 4.3.1. and 4.3.2. The standard error of estimate for room temperature samples (Figure 4.3.1.) is 8.5%. This includes a sampling error of ±5%.
4.4. Desorption efficiency
Desorption Efficiency
of Chloroform from Charcoal with Carbon Disulfide
|
| |||||
| × target conc. | 0.5× | 1× | 2× | ||
|
|
|
| |||
| mg/sample | 0.249 | 0.237 | 0.534 | 0.475 | 1.068 |
|
| |||||
| desorption | 98.3 | 96.1 | 97.4 | 91.5 | 102.2 |
| efficiency, | 92.6 | 94.8 | 100.0 | 94.3 | 106.8 |
| % | 94.2 | 97.6 | 98.3 | 95.7 | 103.9 |
| 93.0 | 83.0 | 96.5 | 95.2 | 96.6 | |
| 95.5 | 96.2 | 100.4 | 95.6 | ||
| 99.4 | 90.1 | 95.8 | 95.3 | ||
| 90.5 | |||||
| 94.8 | |||||
| 90.8 | |||||
| 96.7 | |||||
| 95.5 | 93.0 | 98.1 | 94.2 | 97.3 | |
|
| |||||
5. References
- 5.1. U.S. DHEW/PHS/CDC/NIOSH. Criteria for a Recommended
Standard... Occupational Exposure to Chloroform. HEW (NIOSH), 1974.
5.2. General Industrial Safety and Health Standards OSHA 2206 (29 CFR 1910) Revised January 1976.
5.3. U.S. DHEW/PHS/CDC/NIOSH Current Intelligence Bulletin 9. HEW (NIOSH), March 15, 1976.
5.4. U.S. DHEW/PHS/CDC/NIOSH Occupational Diseases A Guide To Their Recognition. HEW (NIOSH) 77-181, June 1977.
5.5. Patty, Frank A. (editor), Industrial Hygiene and Toxicology. New York: John Wiley and Sons, Inc., Second Revised Edition, 1963.
5.6. Standen, Anthony (ed.), Kirk-Othmer Encyclopedia of Chemical Technology. Second Edition, New York: Interscience Publishers, Volume 5, pp. 119-127, 1964.