1,1,1-TRICHLOROETHANE
| Method no.: | 14 |
| Matrix: | Air |
| Target Concentration: | 350 ppm (1900 mg/m3) OSHA PEL |
| Procedure: | Collection on charcoal adsorbent, desorption with carbon disulfide, analysis by GC using a flame ionization detector. |
| Recommended air volume and sampling rate: |
3 L at 0.2 L/min |
| Reliable quantitation limit: | 0.07 ppm (0.4 mg/m3) |
| Standard error of estimate at the PEL concentration: (Section 4.4.) |
5.2% |
| Status of method: | Evaluated method, This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: January 1980 | Chemist: Duane E. Lee |
Organic Methods Evaluation Branch
OSHA Analytical
Laboratory
Salt Lake City, Utah
1. General Discussion
1.1. Background
1.1.1. History
Outdated methods for the sampling and analysis of
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
The following information is taken directly from Reference 5.3.
1,1,1-Trichloroethane causes central nervous system depression.
A number of human fatalities related to industrial exposure in closed spaces have been reported, some of which may have been "sudden deaths" due to sensitization of the myocardium to epinephrine.
Based on effects caused in monkeys and rats, the following effects are expected in humans: 20,000 ppm for 60 min, coma and possibly death; 10,000 ppm for 30 min, marked incoordination; 2000 ppm for 5 min, disturbance of equilibrium. Human subjects exposed to 900 to 1000 ppm for 20 min experienced lightheadedness, incoordination, and impaired equilibrium; transient eye irritation has also been reported at similar concentrations.
A few scattered reports have indicated mild kidney and liver injury in humans from severe exposure; animal experiments have confirmed the potential for liver, but not for kidney, injury. Skin irritation has occurred from experimental skin exposure to the liquid and from occupational use. The liquid can be absorbed to a moderate degree through the skin.
In dogs, myocardial sensitization to epinephrine occurred at concentrations of 5000 to 10,000 ppm. In a carcinogenicity study, rats and mice were given the liquid orally at two different dose levels, five days a week for 78 weeks. Both female and male test animals exhibited early mortality compared with untreated controls, and a variety of neoplasms was found in both treated animals and controls. Although rats of both sexes demonstrated a positive dose-related trend, no relationship was established between the dosage groups, the species, sex, type of neoplasm, or the sites of occurrence.
The odor threshold has been described by various investigators as ranging from 16 to 400 ppm.
The TLV was set at a level to prevent mild irritation.
1.1.3. Worker exposure
NIOSH estimates that there were 2.9 million workers exposed to
1.1.4. Use and operations where exposure occur
1,1,1-Trichloroethane is mainly used as a solvent for cleaning and other solvent applications. There were 630 million pounds produced in 1976. (Ref. 5.5.)
Industries where
1.1.5. Physical properties (Refs. 5.6. and 5.7.)
| molecular weight: | 133.42 |
| specific gravity: | 1.3249 (26°C/4°C) |
| melting point: | -32.62°C |
| boiling point: | 74.1°C |
| vapor density: | 4.6 (air = 1) |
| vapor pressure: | 127 mm Hg (25°C) |
| color: | colorless liquid |
| refractive index: | 1.43765 (21°C) |
| saturated air: | 16.7% (25°C) |
| saturated air density: | 1.6 (air = 1) |
| solubility: | insoluble in water; soluble in ethanol and ethyl ether |
| molecular formula: | CCl3CH3 |
| synonyms: (Ref. 5.4.) | Aerothene TT; Chloroethene NU; Chlorothene; Chlorothene NU; Chlorothene VG; Chlorten; Inhibisol; methyl chloroform; methyltrichloromethane; NCI-C04626; Alpha-T; trichlorethane; a-trichloroethane. |
1.2. Limit defining parameters
1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 1.2 ng per injection. This is the amount of analyte which will give a well defined peak on the tail from the solvent peak. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 1.2 µg per sample (0.07 ppm or 0.4 mg/m3). This is the amount of analyte spiked on the sampling device which allows recovery of an amount of analyte equivalent to the detection limit of the analytical procedure. (Section 4.1.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 1.2 µg per sample (0.07 ppm or 0.4 mg/m3). This is the smallest amount of analyte which can be quantitated within the requirements of 75% recovery and 95% confidence limits of ±25%. (Section 4.2.)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
1.2.4. Sensitivity
The sensitivity of the analytical procedure over a concentration range representing 0.1 to 2.1 times the PEL concentration based on the recommended air volume is 53430 area units per mg/mL. The sensitivity is determined by the slope of the calibration curve. (Section 4.3.) The sensitivity will vary somewhat with the particular instrument used in the analysis.
1.2.5. Recovery
The recovery of analyte from the collection medium must be 75% or greater. The average recovery over the range of 0.5 to 2 times the PEL is 99.6%. (Section 4.1.)
1.2.6. Precision (analytical method only)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1 and 2 times the PEL concentration is 0.0086. (Section 4.3.)
1.2.7. Precision (overall procedure)
The overall procedure must provide results at the PEL
concentration that are ±25% or better at the 95% confidence level.
The precision at the 95% confidence level for the
1.3. Advantages
1.3.1. The sampling procedure is convenient.
1.3.2. The analytical procedure is quick, sensitive, and reproducible.
1.3.3. Reanalysis of the samples is possible.
1.4. Disadvantages
If other compounds are present, the GC run time must be lengthened so the late eluting peaks will not interfere with the next sample.
2. Sampling Procedure
2.1. Apparatus
2.1.1. An approved and calibrated personal sampling pump whose flow can be determined within ±5% at the recommended flow.
2.1.2. Charcoal tubes: Glass tube, with both ends heat sealed,
7.0 cm × 6-mm i.d. ×
2.2. Reagents
None required.
2.3. Sampling technique
2.3.1. Immediately before sampling, break open the ends of the charcoal tube. All tubes must be from the same lot.
2.3.2. Connect the charcoal tube to the sampling pump with flexible tubing. The short section of the charcoal tube is used as a backup and should be positioned nearer the sampling pump.
2.3.3. The tube should be placed in a vertical position during sampling to minimize channeling.
2.3.4. Air being sampled should not pass through any hose or tubing before entering the charcoal tube.
2.3.5. Seal the charcoal tube with plastic caps immediately after sampling. Also, seal each sample with OSHA sealing tape lengthwise.
2.3.6. With each batch of samples, submit at least one blank tube from the same lot used for samples. This tube should be subjected to exactly the same handling as the samples (break, seal, transport) except that no air is drawn through it.
2.3.7. Transport the samples (and corresponding paperwork) to the lab for analysis.
2.3.8. If bulk samples are submitted for analysis, they should be transported in glass containers with Teflon-lined caps. These samples must not be put in the same container used for the charcoal tubes.
2.4. Breakthrough
Breakthrough tests were run on the primary portion of a charcoal
tube (SKC Lot 107) at a sampling rate of 0.2 L/min from a generated
test atmosphere. The test atmosphere was 708 ppm
2.5. Desorption efficiency
The desorption efficiency from liquid injections on charcoal tubes (SKC Lot 107), averaged 99.6% for 2.98 to 11.9 mg per tube, which is 182 to 728 ppm for a 3-L air volume (Section 4.1.).
2.6. Recommended air volume and sampling rate
2.6.1. The recommended air volume is 3 L.
2.6.2. The recommended sampling rate is 0.2 L/min.
2.6.3. If a longer sampling time is required, the sampling rate should be lowered to 0.1 L/min or 0.05 L/min.
2.7. Interferences (sampling)
2.7.1. At the present time, it is unknown if any compound would
severely interfere with the collection of
2.7.2. Any compound which is suspected of interfering in the collection or analysis should be listed on the sampling data sheet.
2.8. Safety precautions
2.8.1. Safety glasses should be worn when breaking the ends of the tubes.
2.8.2. The broken ends of the tubes should be protected to avoid injury to the person being sampled.
2.8.3. When working in environments containing flammable vapors, do not provide any spark source from equipment used or pumps.
2.8.4. Observe all safety practices for working in hazardous areas.
3. Analytical Procedure
3.1. Apparatus
3.1.1. A GC equipped with a flame ionization detector.
3.1.2. A number of GC columns are available and adequate. The column used for this study was a 10-ft × 1/8-in. stainless steel 7% Penta 100/120 Chrom P AW.
3.1.3. An electronic integrator or other suitable method of measuring peak area.
3.1.4. Two-milliliter vials with Teflon-lined caps.
3.1.5. Microliter syringes, 50-µL for preparing standards, 1-µL for sample injections.
3.1.6. Pipets for diluting standards. A 1-mL pipet for dispensing solvent for desorption, or a 1-mL dispenser pipet.
3.1.7. Volumetric flasks, convenient sizes for preparing standards.
3.2. Reagents
3.2.1. Carbon disulfide, chromatographic grade.
3.2.2.
3.2.3. Purified GC grade helium, hydrogen, and air.
3.3. Standard preparation
3.3.1. Standards are prepared by diluting pure
3.3.2. Forty-five microliters of
3.4. Sample preparation
3.4.1. The front and backup sections of each sample are transferred to separate 2-mL vials.
3.4.2. Each section is desorbed with 1.0 mL of carbon disulfide.
3.4.3. The vials are sealed immediately and allowed to desorb for 30 min with intermittent shaking.
3.5. Analysis
3.5.1. GC conditions
| helium (carrier gas) flow rate: | 25.1 mL/min |
| injector temperature: | 150°C |
| detector temperature: | 200°C |
| column temperature: | 80°C |
| detector: | flame ionization |
| hydrogen flow rate: | 43 mL/min |
| air flow rate: | 248 mL/min |
| injection size: | 1 µL |
3.5.2. Chromatogram (Section 3.5.2.)
3.5.3. Peak areas are measured by an electronic integrator or other suitable means.
3.5.4. An external standard procedure is used. The integrator is calibrated to report results in ppm for a 10-L air sample after correction for desorption efficiency.
3.6. Interferences (analytical)
3.6.1. Any compound having the same general retention time of
3.6.2. GC parameters may be changed to circumvent most interferences.
3.6.3. Retention time on a single column is not considered proof of chemical identity. Samples should be confirmed by GC/MS or other suitable means.
3.7. Calculations
Usually the integrator is programmed to report results in ppm (corrected for desorption efficiency) for a 3-L air sample. The following calculation is used:
| ppm = (A)(3)/B | where | A = ppm on report B = air volume, L |
3.8. Safety precautions
3.8.1. All work using solvents (preparation of standards, desorption of samples, etc.) should be done in a hood.
3.8.2. Avoid any skin contact with all of the solvents.
3.8.3. Safety glasses should be worn throughout the procedure.
4. Backup Data
4.1. Detection limit data
4.1.1. Analytical detection limit
A small amount of analyte (1.2 ng/injection) which still produced
a well defined peak on the tail of the solvent peak was designated
as the analytical detection limit. This was determined with an
analytical standard which contained 0.009 µL of
Reproducibility of the peak, produced by replicate 1.2-ng injections, was good. Twelve injections gave an average analyte peak height of 35 mm with coefficient of variation of 2.4%.
A sample collected from 3 L of air which contained 1.2 ng/µL after desorption with 1 mL of carbon disulfide would represent an air concentration of 0.07 ppm.
4.1.2. Desorption efficiencies for determining the overall detection limit and the reliable quantitation limit
Liquid injections were made on the front portion of charcoal tubes (SKC Lot 107) at 0.0012 to 11.92 mg. These charcoal tubes were refrigerated overnight and desorbed and analyzed the following day. These results are presented in Table 4.1.2. and in Figures 4.1.2.1. and 4.1.2.2. The overall detection limit was determined to be 1.2 µg/sample in Figure 4.1.2.1.
Table 4.1.2.
Desorption Efficiencies for Various
Sampler Loadings
|
| |||||||||
| µg/sample | 11920 | 5960 | 2980 | 1484 | 296.8 | 59.4 | 11.9 | 2.37 | 1.187 |
|
| |||||||||
| desorption | 98.2 | 99.6 | 101.7 | 99.8 | 100.4 | 97.2 | 101.1 | 99.7 | 100.7 |
| efficiency, | 98.5 | 99.5 | 99.4 | 99.0 | 100.2 | 98.0 | 101.0 | -- | 100.0 |
| % | 96.4 | 94.7 | 100.1 | 99.1 | 100.2 | 101.0 | 100.4 | 99.0 | 102.0 |
| 97.3 | 98.6 | 99.2 | |||||||
| 98.2 | 99.5 | 100.3 | The average desorption
efficiency over the range of 2980 to 11920 µg (0.5 to 2 times the target concentration) is 99.6%. | ||||||
| 98.5 | 98.3 | 100.1 | |||||||
| 102.2 | 102.2 | 104.5 | |||||||
| 101.3 | 102.1 | 102.4 | |||||||
| 98.8 | 99.3 | 101.0 | |||||||
|
| |||||||||
4.2. Reliable quantitation limit
The reliable quantitation limit was verified to be the same as the overall detection limit by liquid spiking six samples with loadings equivalent to the overall detection limit (1.187 µg/sample). These samples were analyzed to assure the requirements of at least 75% recovery with a precision (1.96 SD) of at least ±25% were met.
Table 4.2.
Reliable Quantitation Limit
|
| ||||||
| sample no. | % recovered | sample no. | % recovered | |||
|
| ||||||
| 1 2 3 |
98.6 94.3 99.3 |
4 5 6 |
100.0 100.0 98.6 | |||
| ||||||
|
| ||||||
4.3. Precision data
Multiple injections were made of standards that were prepared over a range of 0.1 to 2.1 times the OSHA standard. A standard deviation was determined at each concentration. The pooled coefficient of variation was determined for the range.
Table 4.3.
Analytical Precision
|
| |||||
| × target conc. µg/sample |
0.1× 596 |
0.2× 1190 |
0.5× 2980 |
1.0× 5960 |
2.1× 11920 |
|
| |||||
| area
counts SD CV |
33828 33747 34117 33516 33586 33650 33742 216 0.0064 |
67545 66599 66413 68164 67086 67182 67165 638 0.0095 |
166773 165396 167897 164153 163485 163222 165150 1882 0.0114 |
327123 327763 328157 325401 322931 324246 323908 323029 325319 2117 0.0065 |
647551 643296 642360 635031 634452 633876 639427 5735 0.0090 |
|
| |||||
4.4. Storage data
Samples were collected on charcoal tubes (SKC Lot 107) from a
generated atmosphere containing 354 ppm
Table 4.4.
Storage Tests
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (-4°C to 3°C) | (18.2°C to 21°C) | |||||
|
| |||||||
| 1 3 6 9 12 15 |
102.8 100.6 104.2 102.0 102.2 102.3 |
104.7 99.8 101.6 99.3 101.0 100.8 |
102.5 100.8 102.0 101.6 101.5 101.1 |
106.0 101.0 101.8 105.4 104.3 100.9 |
103.1 103.2 101.5 102.6 103.0 100.0 |
104.4 102.8 103.3 100.9 102.1 101.6 | |
|
| |||||||

Figure 3.5.2. Chromatogram of a standard of

Figure 4.1.1. Chromatogram of the analytical detection
limit of 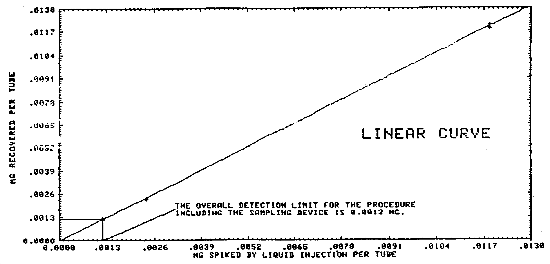
Figure 4.1.2.1. The detection limit of the overall
procedure of 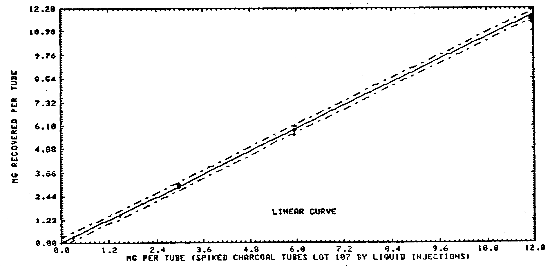
nt face="Arial" size="2">Figure 4.1.2.2. Desorption
efficiency data for 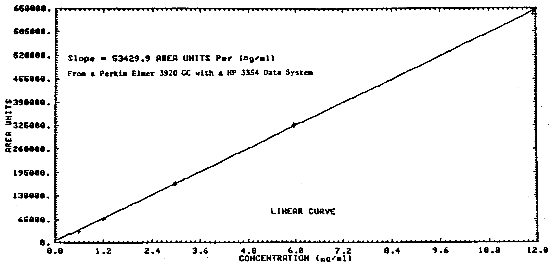
Figure 4.3. Calibration curve of instrument response to
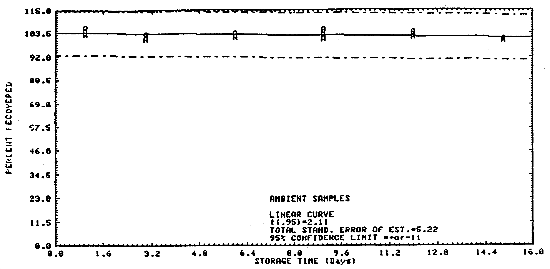
Figure 4.4.1. Ambient storage test of
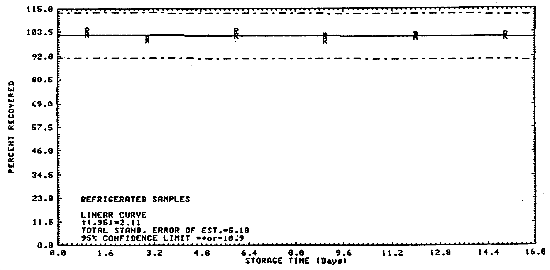
Figure 4.4.2. Refrigerated storage test of
5. References
5.1. American Industrial Hygiene Association: Analytical Abstract "Halogenated Hydrocarbons" A.I.H.A., 1965.
5.2. National Institute for Occupational Safety and Health, U.S. Department of Health, Education, and Welfare: NIOSH Manual of Analytical Methods, Vol. 3, Washington, D.C.: U.S. Government Printing Office, (NIOSH) Pub. No. 77-157-C, Method No. S328, 1977.
5.3. Proctor, N.H., and Hughes, J.P., Chemical Hazards of the Workplace., Philadelphia: J.B. Lippincott Co., pp 488-489, 1978.
5.4. National Institute for Occupational Safety and Health, U.S. Department of Health, Education and Welfare: NIOSH Current Intelligence Bulletin 17. Washington, D.C.: U.S. Government Printing Office (NIOSH) Pub. No. 78-181, 1978.
5.5. A.C.G.I.H.: Methyl chloroform
(
5.6. Standen, A. (editor), Kirk-Othmer Encyclopedia of Chemical Technology. 2nd ed., New York: Interscience Publishers, Vol. 5, pp. 154-157, 1964.
5.7. Patty, F.A. (editor) Industrial Hygiene and Toxicology, 2nd ed., New York: John Wiley and Sons, Inc., p. 1288, 1963.