| Method no.: | 15 |
| Matrix: | Air |
| Target concentration: | 25 ppm (90 mg/m3) (OSHA PEL) |
| Procedure: | Collection on treated Chromosorb 106 tubes, desorption with ethyl acetate, analysis by GC using a flame ionization detector. |
| Detection limit based on recommended air volume: (for analytical procedure only) |
0.27 ppm (0.98 mg/m3) |
| Recommended air volume and sampling rate: |
2 L at 0.2 L/min |
| Coefficient of variation: | 0.023 (for analytical procedure only over the range of 0.54 ppm to 54 ppm based on the recommended air volume) |
| Special requirements: | Chromosorb 106 tubes are pretreated by placing them in a GC oven overnight at 120°C with a carrier gas flowing through them. |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: January 1980 | Chemist: Duane Lee |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
A number of early analytical procedures for nitro paraffins are
reported in the literature (Ref. 5.1.). The only procedure found
which is directed towards industrial hygiene application is a
colorimetric procedure in which the samples are collected in
concentrated sulfuric acid (Ref. 5.2.). The
The most common procedure for determining air concentrations of
solvent vapors is collection of the vapors with charcoal adsorbent
tubes and analysis by GC after desorption with carbon disulfide.
When this procedure is used to determine
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
Exposure to concentrations of
1.1.3. Work population
An estimated 100,000 workers are potentially exposed to
1.1.4. Use and operations where exposure occur
The following information is taken directly from Reference 5.8.
- Solvent systems containing
1.1.5. Physical properties (Refs. 5.9. and 5.10.)
| molecular weight: | 89.09 |
| density at 25°C: | 0.98290 g/mL |
| melting point: | -91.32°C |
| boiling point: | 120°C |
| vapor pressure at 20°C: | 12.9 mm Hg |
| vapor density (air = 1): | 3.06 |
| flash point: | 103°F |
| refractive index: | 1.39439 |
| critical temperature: | 344°C |
| ignition temperature: | 802°F |
| lower flammability limit (% by vol. in air): |
2.6 |
| solubility at 25°C,(% by wt compound in water): |
1.7 |
| wt water in compound: | 0.5 |
| molecular structure: | |
| other names: | dimethylnitromethane; isonitropropane; 2-NP; nitroisopropane |
1.2. Detection limit, precision, sensitivity and working range
- 1.2.1. The detection limit for the analytical procedure is 2 ng
per injection with a coefficient of variation of 0.055 at this
level. (Section 4.1.) The detection limit was determined using
1.0-µL injections.
1.2.2. The pooled coefficient of variation of the analytical procedure over the range of 3.95 to 395 µg per sample is 0.023. (Section 4.2.) This represents an air concentration range of 0.54 to 54 ppm based on the recommended sampling and analytical procedures.
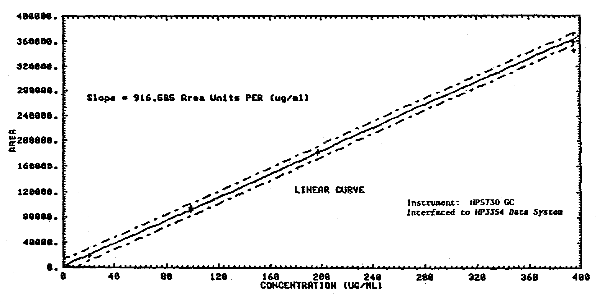
1.2.3. The sensitivity of the analytical procedure over a concentration range representing 0.02 to 2 times the target concentration based on the recommended air volume is 916.6 area units per µg/mL. The sensitivity is determined by the slope of the calibration curve (Section 4.2.) The sensitivity will vary somewhat with the particular instrumentation used in the analysis.
1.2.4. The lower limit of the estimated working range, assuming adequate desorption efficiency, is 0.27 ppm. The upper limit of the working range is dependent on the capacity of the collection medium.
1.3. Accuracy
- 1.3.1. The overall procedure must provide results that are
within 25% or better at the 95% confidence interval.
1.3.2. The recovery of analyte from the collection medium after storage must be 75% or greater.
1.3.3. The overall procedure has met the above validation criteria. (Section 4.3.)
1.4. Advantages
- 1.4.1. The sampling procedure is convenient.
1.4.2. The analytical procedure is quick, sensitive, and reproducible.
1.5. Disadvantages
If other compounds are present, the GC run time must be lengthened so the late eluters will not interfere with the next sample.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. An approved and calibrated personal sampling pump whose
flow can be determined within ±5% at the recommended flow.
2.1.2. Chromosorb 106 tubes: Glass tube with both ends heat sealed, 7 cm × 6-mm o.d. × 4-mm i.d., containing 100-mg front and 50-mg backup sections of Chromosorb 106 (60/80 mesh). SKC tubes or equivalent.
2.1.3. The Chromosorb 106 tubes are conditioned prior to use by heating overnight in a GC oven at 120°C with a carrier gas of helium or nitrogen flowing through the tubes. Conditioning removes residual components from the Chromosorb 106 which may interfere with the analysis.
2.2. Reagents
None required.
2.3. Sampling technique
- 2.3.1. Immediately before sampling, remove the caps of the
Chromosorb 106 tube. All tubes must be from the same lot.
2.3.2. Connect the Chromosorb 106 tube to the sampling pump with flexible tubing. The short section of the Chromosorb 106 tube is used as a backup and should be positioned nearer the sampling pump.
2.3.3. The tube should be placed in a vertical position during sampling to minimize channeling.
2.3.4. Air being sampled should not pass through any hose or tubing before entering the Chromosorb 106 tube.
2.3.5. Seal the Chromosorb 106 tube with plastic caps immediately after sampling. Also, seal each sample with OSHA sealing tape lengthwise.
2.3.6. With each batch of samples, submit at least one blank tube from the same lot used for samples. This tube should be subjected to exactly the same handling as the samples (open, seal, transport) except that no air is drawn through it.
2.3.7. Transport the samples (and corresponding paperwork) to the lab for analysis.
2.3.8. If bulk samples are submitted for analysis, they should be transported in glass containers with Teflon-lined caps. These samples must not be put in the same container used for the Chromosorb 106 tubes.
2.4. Breakthrough
Breakthrough tests were performed with the primary section of
Chromosorb 106 tubes using a controlled test atmosphere containing 52
ppm
2.5. Desorption efficiency
The desorption efficiency from liquid injections on Chromosorb 106 tubes averaged 99.3% for 99 to 395 µg per tube, which is 13.6 to 54 ppm for a 2-L air volume. (Section 4.4.)
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 2 L.
2.6.2. The recommended sampling rate is 0.2 L/min.
2.6.3. If a longer sampling time is required, a sampling rate of 0.05 L/min to 0.1 L/min can be used.
2.7. Interferences (sampling)
- 2.7.1. At the present time, it is unknown if any compound would
severely interfere with the collection of
2.7.2. Any compound which is suspected of interfering in the collection or analysis should be listed on the sampling data sheet.
2.8. Safety precautions
- 2.8.1. The broken ends of the tubes should be protected to avoid
injury to the person being sampled.
2.8.2. When working in environments containing flammable vapors, do not provide any spark source from equipment used or pumps.
2.8.3. Observe all safety practices for working in hazardous areas.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A GC equipped with a flame ionization detector.
3.1.2. A number of GC columns are available and adequate. The column used for this study was a 1/8-in. × 10-ft, stainless steel, 7% Tetracyanoethylated Pentaerythritol on 100/120 mesh Chromosorb P-AW.
3.1.3. An electronic integrator or other suitable method of measuring peak areas.
3.1.4. Two-milliliter vials with Teflon-lined caps.
3.1.5. Microliter syringes, 10-µL for preparing standards, 1-µL for sample injections.
3.1.6. Pipets for diluting standards. A 1.0-mL pipet for dispensing solvent for desorption, or a 1.0-mL repipet dispenser.
3.1.7. Volumetric flasks, convenient sizes for preparing standards.
3.2. Reagents
- 3.2.1. Ethyl acetate, chromatographic grade.
3.2.2.
3.2.3. Purified GC grade helium, hydrogen, and air.
3.3. Standard preparation
- 3.3.1. Standards are prepared by diluting pure
3.3.2. Five microliters of
3.4. Sample preparation
- 3.4.1. The front and back sections of each sample are
transferred to separate 2-mL vials.
3.4.2. Each section is desorbed with 1.0 mL of ethyl acetate.
3.4.3. The vials are sealed immediately and allowed to desorb for 30 min with intermittent shaking.
3.5. Analysis
- 3.5.1. GC conditions:
| helium (carrier gas) flow rate: | 25 mL/min |
| detector (flame ionization) | |
| hydrogen flow: | 30 mL/min |
| air flow: | 240 mL/min |
| injector temperature: | 150°C |
| detector temperature: | 250°C |
| column temperature: | 100°C |
| injection size: | 1.0 µL |
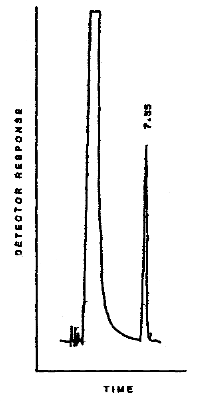
3.5.2. Chromatogram
A typical chromatogram of
3.5.3. Peak areas are measured by an electronic integrator or other suitable means.
3.5.4. An external standard procedure is used. The integrator is calibrated to report results in ppm for a 2-L air sample after correction for desorption efficiency.
3.6. Interferences (analytical)
- 3.6.1. Any compound having the same general retention time as
3.6.2. GC parameters may be changed to circumvent most interferences.
3.6.3. Retention time on a single column is not considered proof of chemical identity. Samples should be confirmed by GC/MS or other suitable means.
3.7. Calculations
Usually the integrator is programmed to report for a 2-L air sample. The following calculation is used:
| ppm = A/(B/2) | where | A = ppm on report B = air volume (L) |
3.8. Safety precautions
- 3.8.1. All work using solvents (preparation of standards,
desorption of samples, etc.) should be done in a hood.
3.8.2. Avoid any skin contact with all of the solvents.
3.8.3. Safety glasses should be worn throughout the procedure.
4. Backup Data
- 4.1. Detection limit
A small amount of analyte (1.98 ng/injection) which produced a well
defined peak was designated as the analytical detection limit. This
was determined with an analytical standard which contained 0.002 µL of
Reproducibility of the peak produced by 1.98 ng was good. Eight injections resulted in a coefficient of variation of 5.5%.
A sample collected from 2 L of air which contained 1.98 ng/µL after desorption with 1 mL of ethyl acetate would represent an air concentration of 0.27 ppm.
4.2. Precision data
Multiple injections were made of the standards that were prepared over a range of 0.02 to 2.2 times the target concentration. A standard deviation and a coefficient of variation was determined at each concentration. The pooled coefficient of variation was determined for the range.
Analytical Precision
|
| |||||
| × target conc. µg/sample |
0.02× 3.95 |
0.11× 19.57 |
0.54× 98.76 |
1.1× 197.52 |
2.2× 395.04 |
|
| |||||
| area counts SD CV |
3785 3620 3814 3672 3723 92 0.0247 |
19266 19397 19095 19126 19221 139 0.0072 |
93540 93869 96997 91388 93948 2311 0.0246 |
183170 185086 182714 186600 184393 1795 0.0097 |
372497 372586 360046 345140 362567 13026 0.0359 |
|
| |||||
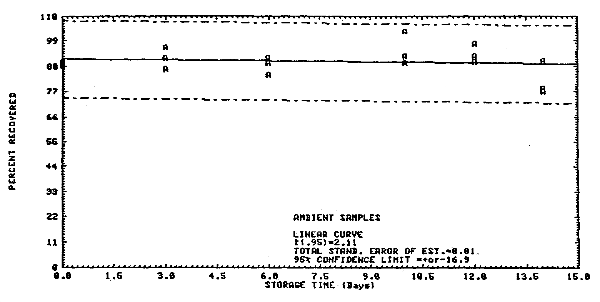
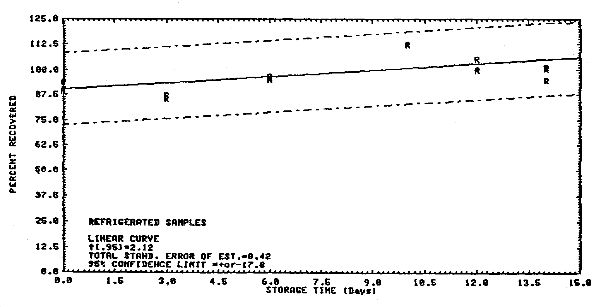
4.3. Storage data
Samples were collected on Chromosorb 106 from a generated
atmosphere containing 25.3 ppm
Storage Tests
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (-1°C to 6°C) | (21.3°C to 23°C) | |||||
|
| |||||||
| 1 3 6 10 12 14 |
89.6 85.8 96.2 112.4 99.7 101.4 |
90.4 lost 95.0 112.9 100.0 94.7 |
94.0 87.2 96.8 112.3 104.9 100.6 |
88.9 86.9 84.4 103.4 90.0 90.6 |
88.5 91.9 91.8 92.4 92.6 77.2 |
90.0 96.5 89.4 89.7 97.8 78.8 | |
|
| |||||||
4.4. Desorption efficiency
Liquid injections were made on the front section of conditioned Chromosorb 106 tubes at approximately 0.5, 1, and 2 times the target concentration. These were refrigerated overnight, desorbed and analyzed the following day. The overall desorption for the concentration studied is 99.3%.
Desorption Efficiency
|
| |||
| × target conc. µg/sample |
0.5× 98.76 |
1× 197.5 |
2× 395.0 |
|
| |||
| desorption efficiency, % |
111.7 104.9 92.8 103.7 106.6 102.7 |
98.4 100.4 99.2 92.1 95.1 97.7 |
97.0 97.2 95.7 95.9 99.0 97.5 |
|
| |||
5. References
- 5.1. Standen, A. (Ed.), "Kirk-Othmer Encyclopedia of Chemical
Technology". 2nd ed., New York: Interscience Publishers, Vol. 13, pp
875-876, 1964.
5.2. Jones, L. R., American Industrial Hygiene Association Journal, 24, 11-16, 1963.
5.3. Taylor, D. G. (Manual Coordinator) "NIOSH Manual of Analytical Methods". 2nd Edition, U.S. DHEW/PHS/CDC/NIOSH, Vol. 4, Method No. P&CAM 272, 1978.
5.4. Skinner, J. B., Ind. Med., 16, 441, 1974.
5.5. "Documentation of Threshold Limit Values", American Conference of Governmental Industrial Hygienists, 3rd Ed., p. 188-189, 1971.
5.6. Freon and Dutra Arch. Ind. Hyg. and Occ. Med., 5, 52, 1952.
5.7. Gaultier, M., et.al., Arch. I. Mal. Prof., 25, 425, 1964.
5.8. U.S. DHEW/PHS/CDC/NIOSH "Current Intelligence Bulletin 17" DHEW (NIOSH), April 25, 1977.
5.9. Patty, F. A. (Ed.) "Industrial Hygiene and Toxicology", 2nd ed., New York: John Wiley and Sons, Inc., p. 2073, 1963.
5.10. Standen, A. (Ed.), "Kirk-Othmer Encyclopedia of Chemical Technology". 2nd ed., New York: Interscience Publishers, Vol. 13, p. 866, 1964.