2,4-TDI and MDI
| Method no.: | 18 |
| Matrix: | Air |
| OSHA Standard: | The current ceiling PEL for 2,4-toluene diisocyanate
( |
| Procedure: | Collection in a bubbler containing nitro reagent in toluene and analysis by high pressure liquid chromatography. |
| Recommended air volume and sampling rate: |
20 L at 1 L/min |
| Detection limit of the overall procedure: |
0.15 ppb (1 µg/m3) for 0.10 ppb (1 µg/m3) for MDI |
| Reliable quantitation limit: | 0.3 ppb (2.4 µg/m3) for
0.3 ppb (3.4 µg/m3) for MDI |
| Standard error of estimate at the ceiling PEL: |
5.5% for both |
| Status: | Sampling and analytical method which has been evaluated as closely as possible to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: February 1980 | Chemist: Kevin Cummins |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
Analysis of aromatic diisocyanates is frequently performed by
employing the Marcali or the Ranta colorimetric methods. In the
past, the Marcali method has been the method of choice for the
analysis of diisocyanates by the OSHA laboratory. This method
employs an acidified aqueous bubbler solution to trap and convert
the diisocyanates into their respective diamines. Diazotization and
coupling of the diamines with
Collection and derivatization of diisocyanates in nitro reagent
(0.0002 M
1.1.2. Toxic effects (This section is for information only and should not be taken as a basis for OSHA policy.)
Isocyanates are strong irritants and can produce an acute allergic response among some individuals. There is no data to indicate that diisocyanates are carcinogenic or teratogenic. (Ref. 5.1.) Industry sources indicate that diisocyanates yielded negative results by the Ames test for mutagenicity. (Ref. 5.4.) However, another study reported MDI to be mutagenic to Salmonella Typhimurium in the presence of a mammalian liver activating system, although TDI and dicyclohexylmethane 4,4'-diisocyanate showed no mutagenic activity. The NIOSH criteria document states that in the absence of supporting data, this study is insufficient evidence that diisocyanates are mutagens. (Ref. 5.1.)
1.1.3. Operations where exposure occurs
The aromatic diisocyanates TDI and MDI are widely used in the production of polyurethane foams and elastomers. Prepolymer forms of both aromatic and aliphatic diisocyanates are widely employed in the polyurethane coatings industry. (Ref. 5.5.) These prepolymer forms, while generally non-volatile, may contain varying amounts of free diisocyanates. (Ref. 5.6., 5.7.) Because of the many applications for diisocyanates, the potential for exposure is widespread.
1.1.4. Size of work population that are exposed
A 1972-74 NIOSH survey estimates that 50,000-100,000 employees in the United States were potentially exposed to diisocyanates. NIOSH indicates that this number does not include occasional users of isocyanate preparations and may underestimate the number of workers exposed. (Ref. 5.1.)
1.1.5. Physical properties (Refs. 5.8.- 5.9.)
| toluene 2,4-diisocyanate | C9H6N2O2 |
| molecular weight | 174.15 |
| melting point | 19.5-21.5°C |
| flash point | 152°C |
| solubility | dissolves in most organic solvents |
| structure | Figure 1.1.5. |
| odor | sharp, pungent, irritating |
| reacts with water to generate CO2 | |
| 4,4'-methylenebisphenyl diisocyanate (diphenylmethane 4,4'-diisocyanate) MDI C15H10N2O2 | |
| molecular weight | 250 |
| melting point | 37°C |
| specific gravity | 1.97 (70°C) |
| structure | Figure 1.1.5. |
1.2. Limit defining parameters
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 0.3 ng per
injection for both
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 0.02 µg per
sample for both
1.2.3. Reliable quantitation limit
Recoveries for both
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Sensitivity
The sensitivity of the reverse phase HPLC method over the 0.5× to
2× PEL range for
1.2.5. Recovery
Recovery was determined to be 100% over the 0.5× to 2× PEL range
for both
1.2.6. Precision for the analytical procedure
The pooled coefficient of variation was determined for both the
reverse phase and the normal phase HPLC methods. Ten-milliliter
aliquots of 0.0002 M nitro reagent in toluene placed in evaporator
tubes were spiked with a mixture of
1.2.7. Storage
To determine the stability of the derivatized diisocyanates, 36 samples were prepared in the following manner, and analyzed over a 15-day period:
Twenty liters of air at 17°C and approximate relative humidity of
73% were pulled through 36 individual bubblers, each containing 15
mL of 0.0002 M nitro reagent in toluene. Each bubbler was then
spiked with 3.02 µg
1.3. Advantages
- 1.3.1. The sampling and analytical procedure is specific and
sensitive for a wide range of isocyanates and diisocyanates employed
in industry.
1.3.2. Samples are stable at room temperature, requiring no special storage requirements.
1.3.3. Reanalysis of samples is possible.
1.3.4. Multiple analysis of other isocyanates or diisocyanates is possible.
1.3.5. Analysis by two different HPLC methods for the same sample enables the analyst to gather additional confirmatory information.
1.4. Disadvantages
- 1.4.1. The use of toluene nitro reagent in bubblers for sampling
is both cumbersome and potentially hazardous.
1.4.2. Although the collecting solution is stable for at least one month if stored in a refrigerator in a dark bottle, proper care must be taken to insure its integrity. Prolonged exposure to light or heat may result in degradation of the nitro reagent.
2. Sampling Procedure
NOTE: Due to laboratory limitations and the potentially hazardous nature of isocyanates, a complete evaluation of the sampling procedure was not possible. The following sampling data represent a partial evaluation of the sample technique based on these limitations.
- 2.1. Apparatus
- 2.1.1. An approved and calibrated sampling pump whose flow can
be determined within ±5% at the recommended flow.
2.1.2. Clean, dry 25-mL glass bubblers, fitted with ground glass joints and a fritted glass inlet.
2.1.3. Clean, dry, glass scintillation vials fitted with leakproof Polyseal caps or other suitable glass containers for shipping samples.
2.1.4. Disposable Pasteur type glass pipettes with rubber bulb for transferring collection solution.
2.2. Reagents
- 2.2.1. Dry HPLC grade or UV grade toluene.
2.2.2. Coconut shell charcoal, 6-14 mesh.
2.2.3. Nitro reagent collection solution.
Nitro reagent (p-nitrobenzyl-N-n-propylamine hydrochloride) is commercially available from Regis Chemical Company. To prepare the collecting solution, dissolve 120 mg of the reagent in approximately 10 mL of HPLC grade water. Precipitate the free amine with 13 mL of 1 N NaOH. Extract the aqueous solution with HPLC grade toluene, and dry the toluene extract with anhydrous Na2SO4. Dilute the solution with toluene to 250 mL and store refrigerated in a dark bottle. The resulting solution is 0.002 M nitro reagent in toluene. Dilute 10 fold with toluene for use as collecting solution.
2.3. Sampling technique
- 2.3.1. Approximately 15 mL of 0.0002 M nitro reagent in toluene
is placed in a clean, dry glass bubbler for sampling. The analytical
procedure has enough sensitivity to permit sampling a minimum of 1 L
of air. In routine sampling, however, 10 to 20 L of air is
recommended. The recommended sampling rate is 1 L/min.
2.3.2. The 15 mL of toluene reagent will permit sampling for
approximately 1 h at 1 L/min. If excess evaporation does occur
during sampling, sampling must be interrupted and dry HPLC grade
toluene added to the bubbler before resuming. Under normal
conditions, it should not be necessary to add more toluene nitro
reagent to the bubbler. The reagent is in sufficient excess such
that an original 15 mL volume of the 0.0002 M nitro reagent in
toluene contains sufficient reactive amine to permit sampling for 8
h at 1 L/min of a work atmosphere that is 15 times in excess of the
0.005 ppm TWA PEL for either
2.3.3. It is recommended that a glass bubbler filled with approximately 16 grams of coconut shell charcoal be placed after the sampling bubbler to trap toluene vapors. This system effectively traps toluene vapors for 1 h at a flow rate of 1 L/min at 25°C. The sampling pump must be calibrated with both the charcoal trap and the toluene nitro reagent bubbler connected in series to insure an accurate flow rate.
2.3.4. The use of tubing or cassettes in front of the inlet to the bubbler must be avoided, since diisocyanates are readily trapped by these materials (Ref. 5.10.).
2.3.5. After sampling, the collecting solution is transferred to a glass vial for shipping. Rinse the inlet tube and bubbler assembly with several 1-mL portions of toluene using a disposable Pasteur pipet. Transfer these rinses to the shipping vial.
2.3.6. Insure that the container is leakproof, and seal with the properly labeled OSHA seal.
2.3.7. Bulk samples submitted for analysis must be shipped in sealed vials and in a separate container.
2.4. Breakthrough
- 2.4.1. Experimental design
Due to present laboratory limitations, test atmospheres of diisocyanates cannot effectively be generated for the determination of reliable collection efficiencies. However, the following preliminary studies were done to approximate a test atmosphere.
Glass bubblers for sampling were fitted with a short piece of
silanized glass tubing containing a portion of silanized glass wool.
The glass tubing was butted to the inlet of the bubbler using a
short piece of plastic tubing. In this manner, the exposed surface
area of the plastic tubing was minimized. The glass wool was then
spiked with amounts of
Air sampling was performed on a three-sampling-port manifold equipped with a probe to monitor humidity. The inlet of the bubbler, fitted with the glass tubing containing spiked glass wool, was attached to the sampling port. The outlet of the bubbler was attached to a vacuum pump. A critical orifice between the bubbler and the pump maintained a constant 1 L/min flow rate.
Dry air samples were prepared by attaching a drying tube to the manifold inlet. Humid air samples were generated by passing air through water in a controlled temperature water bath. The humidity was monitored in the sampling manifold via the humidity probe.
2.4.2. Breakthrough results
Recoveries of triplicate 1.51, 3.02, and 5.85 µg spikes of
Recoveries for MDI spikes in the 0.5 to 2 times the PEL range were zero for both dry and humid air. This is not unexpected since MDI is a solid at room temperature, and is not volatile under these conditions.
2.4.3. Breakthrough discussion
These results indicate that nitro collecting solution effectively
captures volatile
Surprisingly, no significant loss due to humid air was observed
in this study. This may be due to a rapid volatilization and
absorption in the collecting solution. In effect, the
The data gathered in this report indicate that hydrolysis of the
diisocyanates,
2.5. Recommended air volume and sampling rate
- 2.5.1. The recommended air volume is 20 L.
2.5.2. The recommended sampling rate is 1 L/min.
2.6. Interferences
- 2.6.1. Compounds that can react with an isocyanate represent a
potential interference. These would include molecules containing the
following functional groups: amines, alcohols, phenols, carboxylic
acids, and sulfhydryls.
2.6.2. Strong oxidizing agents can potentially destroy the nitro reagent collecting solution.
2.7. Safety precautions
Care must be exercised in sampling with bubblers containing toluene since it is a highly flammable solvent. Sampling around open flames or while smoking must be avoided.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. High pressure liquid chromatograph equipped with UV
detector, manual or automatic sample injector, and chart recorder.
3.1.2. HPLC stainless steel columns capable of separating diisocyanate derivatives. Columns employed in this study were a 30-cm × 3.9-mm i.d. stainless steel column slurry packed with 10 µm C18 nucleosil (Macherey-Nagel, Duren, W. Germany) and a Waters 30-cm × 3.9-mm i.d. µBondapak CN column.
3.1.3. An electronic integrator, or some other suitable method of determining peak areas.
3.1.4. Small volume (1-4 mL) vials for storage and analysis of samples.
3.1.5. Microliter syringes (10-100 µL) for sample injection.
3.1.6. Temperature controlled water bath equipped with nitrogen stream drying needles.
3.1.7. Evaporator tubes, 10 mL or larger.
3.1.8. Rotary evaporator for stripping off organic solvents.
3.1.9. Volumetric pipettes and flasks for preparation of standards.
3.2.10. Suitable glassware for preparation of nitro reagent and for preparation of diisocyanate urea derivatives.
3.2. Reagents
- 3.2.1. Reagent grade phosphoric acid.
3.2.2. 1 N NaOH.
3.2.3. HPLC grade methanol, n-heptane, toluene, methylene chloride, isopropanol, hexane, acetonitrile, and isooctane.
3.2.4. HPLC grade water. Our laboratory employs a commercially available water filtration system for the preparation of HPLC grade water.
3.2.5.
3.2.6. Recrystallized MDI.
3.2.7. p-Nitrobenzyl-N-n-propylamine hydrochloride from Regis Chemicals, Morton Grove, IL.
3.3. Standard preparation
- 3.3.1. Recrystallization of MDI.
MDI for derivative formation is recrystallized according to the procedure of Vogt (Ref. 5.12.): 5 g of MDI is dissolved in 30 mL of methylene chloride and filtered. The undissolved residue is discarded. The methylene chloride solution is concentrated to 5 to 10 mL. A small amount of n-heptane is added to start precipitation. The MDI precipitate is filtered and dried under vacuum.
3.3.2. The diisocyanate urea derivatives for use as standards are prepared according to the method of Vogt (Ref. 5.12.)
One gram of p-nitrobenzyl-N-n-propylamine hydrochloride is
extracted into 50 mL of toluene as described in Section 2.2.3. for
the preparation of collecting solution. A solution of 0.31 g/30 mL
of
MDI urea derivative preparation:
Two and four-tenths grams of p-nitrobenzyl-N-n-propylamine hydrochloride is extracted into 50 mL of n-heptane in a manner analogous to the preparation of nitro collecting solution (Section 2.2.3.). One and three-tenths grams recrystallized MDI/25 mL methylene chloride solution is slowly added to the heptane nitro reagent solution with stirring (molar excess 2.1 to 1). The precipitated MDI urea derivative is filtered and washed with methylene chloride. This derivative can be recrystallized by redissolving in methylene chloride and reprecipitating with n-heptane. Yield is approximately 1 g.
3.3.3. Preparation of working range standards
A stock standard solution is prepared by dissolving
| MW 2,4-TDI
MW 2,4-TDI urea |
= | 174
562 |
= 0.310 |
Similarly, the correction factor for MDI urea is 0.392
| MW MDI
MW MDI urea |
= | 250
638 |
= 0.392 |
If the urea derivatives of the diisocyanates are not available, working range standards of the derivatives can be prepared from nitro reagent and the free diisocyanate standard. The following is a suggested method for their preparation:
Prepare a stock solution of the diisocyanate in the 0.1 to 0.5 mg/mL range by dissolving the diisocyanate in dry methylene chloride. Aliquots of this stock solution are then reacted with a twofold molar excess of nitro reagent in methylene chloride. (Nitro reagent in methylene chloride is prepared in the same manner as nitro collecting solution, Section 2.2.3. by substituting methylene chloride for toluene.)
For normal phase analysis, the derivatized diisocyanate in methylene chloride can be used as a standard. For reverse phase analysis, the methylene chloride is evaporated, and the diisocyanate urea derivative formed is redissolved in acetonitrile.
3.4. Sample preparation
- 3.4.1. The entire volume of the toluene nitro collecting
solution is transferred to a
3.4.2. The dried sample is redissolved in several milliliters of methylene chloride and the solution is transferred to a 10-mL evaporator tube. Several additional small volumes of methylene chloride are used to thoroughly rinse the flask. The sample in the evaporator tube is taken to dryness in a 45°C water bath equipped with N2 stream drying needles.
3.4.3. For normal phase HPLC analysis, exactly 1 mL of methylene chloride is pipetted into the tube and the sample is thoroughly vortexed. The solution is then transferred to a small capped vial for analysis. For reverse phase analysis, 1 mL of acetonitrile is used in place of methylene chloride.
3.5. Analysis
Note: The recommended analytical method for isocyanates is the reverse phase method. The normal phase method is included as an alternate method.
- 3.5.1. Reverse Phase HPLC Conditions (Figure 4.7.1.)
| column: | 30-cm × 3.9-mm stainless steel column, slurry packed with 10L C18 Nucleosil (Macherey-Nagel; Duren, W. Germany) or suitable replacement. |
| mobile phase: | 73/26.9/0.1 methanol/water/phosphoric acid (v/v/v) |
| flow rate: | 1 mL/min |
| UV detector: | 254 nm |
| injection size: | 10-30 µL |
3.5.2. Normal phase HPLC conditions (Figure 4.7.2.)
| column: | Waters µBondapak CN, 30 cm × 3.9 mm or a suitable replacement. |
| mobile phase: | isooctane/isopropanol/methanol 75/10/15 (v/v/v) |
| flow rate: | 1 mL/min |
| UV detector: | 254 nm |
| injection size: | 25 µL |
3.5.3. Analysis discussion
In the past, the normal phase method developed in our laboratory was employed. The reverse phase method now recommended is an adaptation of a method developed by Sango. (Ref. 5.13.) The use of acid in the mobile phase serves to protonate the secondary amine of the excess nitro reagent and causes it to elute with the solvent front. For some types of reverse phase packing materials, it is necessary to make the methanol/water mobile phase 1% in triethylamine and adjust the pH to 3 with phosphoric acid as described by Sango. The binding of the nitro reagent to the column may be caused by an interaction of the amine with exposed silica sites. With some packing materials, a simple pH adjustment serves to elute the nitro reagent, whereas in other cases it is necessary to effectively coat all the active binding sites using a non-UV absorbing protonated tertiary amine in the mobile phase. Spherisorb 10-µm ODS, and Waters Radial Pak A (reverse phase), both require triethylamine in the mobile phase, while Waters µBondapak C18 and Nucleosil C18 do not.
It should also be recognized that in the normal phase method, the nitro reagent does not elute early. Late elution can interfere with the routine sample analysis and also cause baseline drift problems.
Finally, Figure 4.7.3. demonstrates the separation of 2,4- and 2,6-TDI isomers by reverse phase. We have not achieved this separation using the CN column, however, it is easily accomplished using reverse phase techniques. Since industrial applications employ an isomeric mixture of 2,4- and 2,6-TDI, the ability to achieve the separation is important.
3.6. Interferences
- 3.6.1. Any compound having the same retention time as the
standards is a possible interference. Generally, chromatographic
conditions can be altered to separate an interference. The reverse
phase method is especially flexible in this regard since it is more
selective for isocyanate-urea derivatives than the normal phase
method.
3.6.2. Retention time data on a single column is not proof of
chemical identity. Analysis by alternate column system, ratioing of
wavelength response using a dual channel UV detector, and ultimately
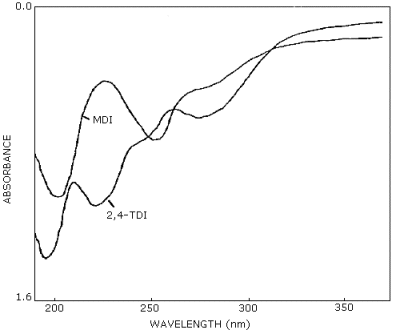
mass spectrometry are additional means of identity. (See UV spectra
for
3.7. Calculations
The concentration in µg/mL of
| (µg/mL diisocyanate) (1 mL)
air volume, L |
× | 1000 L
1 m3 |
= | µg
m3 |
3.8. Safety precautions
- 3.8.1. Sample and standard preparations should be done in a
hood. Avoid exposure to the diisocyanate standards.
3.8.2. Avoid skin contact with all solvents.
3.8.3. Wear safety glasses at all times.
4. Backup Data
- 4.1. Detection limit of the analytical procedure
The detection limit for the analytical procedure was determined by
injecting 30 µL of 0.012 µg/mL
4.2. Detection limit of the overall procedure
The detection limit of the overall procedure is determined to be the amount of analyte spiked in a bubbler which can be detected at the analytical detection limit.
Detection Limit Data
|
| |||
| 2,4-TDI (µg) | MDI (µg) | ||
|
|
| ||
| amount spiked |
amount recovered |
amount spiked |
amount recovered |
|
| |||
| 0.017 0.048 0.048 0.096 0.096 0.190 0.190 |
0.016 0.042 0.042 0.096 0.075 0.185 0.189 |
0.028 0.069 0.069 0.14 0.14 0.28 0.28 |
0.029 0.056 0.074 0.12 0.12 0.28 0.27 |
|
| |||
Linear plots of the data in Table 4.2. result in an overall
detection limit of 0.02 µg per sample for both
4.3. Reliable quantitation limit
The data presented below shows the % recovery and precision for
samplers spiked with 0.048 µg of
RQL Data for
|
| |||
| % recovery | |||
|
| |||
| 87.5 87.5 100.0 100.0 87.5 |
SD 1,96(SD) |
= = = |
92.5 6.8 13.3 |
|
| |||
The data presented below shows the % recovery and precision for samplers spiked with 0.069 µg of MDI, the reliable quantitation limit.
RQL Data for MDI
|
| |||
| % recovery | |||
|
| |||
| 81.2 107.0 98.6 95.6 95.6 82.6 |
SD 1,96(SD) |
= = = |
93.4 9.9 19.4 |
|
| |||
4.4. Sensitivity
|
| ||||
| 0.549 µg/mL | 1.10 µg/mL | 2.20 µg/mL | 5.49 µg/mL | 10.98 µg/mL |
|
| ||||
| 70,967 76,069 |
149,529 168,730 157,975 155,228 |
314,329 319,263 313,749 311,942 |
761,687 773,831 773,433 |
1,516,850 1,514,600 |
|
| ||||
MDI Standards in Acetonitrile and Their Area Response
|
| ||||
| 0.588 µg/mL | 1.18 µg/mL | 2.35 µg/mL | 5.88 µg/mL | 11.77 µg/mL |
|
| ||||
| 109,858 |
230,223 235,266 235,240 235,413 |
472,616 459,047 463,450 467,982 |
1,160,002 1,135,657 1,142,479 |
2,306,632 2,307,841 |
|
| ||||
The calibration curves for
4.5. Precision and recovery
Precision and Recovery Data
for
|
| |||
| × PEL µg/sample |
0.5× 1.44 |
1× 2.89 |
2× 5.78 |
|
| |||
| µg
recovered SD CV |
1.41 1.40 1.45 1.48 1.45 1.39 1.43 0.035 0.025 |
2.90 2.95 2.87 2.92 2.90 2.97 2.92 0.037 0.013 |
5.73 5.75 5.80 5.74 5.81 5.80 0.036 0.063 |
|
| |||
Precision and Recovery Data
for MDI Using Reverse phase HPLC
|
| |||
| × PEL µg/sample |
0.5× 2.40 |
1× 4.89 |
2× 9.78 |
|
| |||
| µg
recovered SD CV |
2.36 2.37 2.39 2.39 2.34 2.57 2.40 0.084 0.035 |
4.78 4.85 4.71 4.80 4.83 5.00 4.83 0.097 0.020 |
9.40 9.41 9.53 9.77 9.47 9.77 9.56 0.17 0.018 |
|
| |||
Precision and Recovery Data
for
|
| |||
| × PEL µg/sample |
0.5× 1.44 |
1× 2.89 |
2× 5.78 |
|
| |||
| µg
recovered SD CV |
1.52 1.42 1.42 1.40 1.50 1.33 1.37 1.52 1.52 1.49 1.45 0.070 0.048 |
3.02 2.87 2.86 2.96 3.12 3.12 2.98 2.95 2.99 3.03 2.96 0.080 0.027 |
5.52 5.59 5.84 5.97 5.99 6.07 6.13 6.22 6.24 5.95 0.25 0.043 |
|
| |||
Precision and Recovery Data
for MDI Using Normal Phase HPLC
|
| |||
| × PEL µg/sample |
0.5× 2.40 |
1× 4.89 |
2× 9.78 |
|
| |||
| µg
recovered SD CV |
1.54 2.55 2.39 2.30 2.58 2.20 2.26 2.60 2.60 2.45 0.15 0.062 |
5.09 4.79 5.05 4.95 5.23 5.00 5.03 5.20 5.07 5.05 0.13 0.026 |
9.18 9.31 9.70 9.70 9.95 10.02 10.35 10.39 2.51 9.92 0.44 0.044 |
|
| |||
These results indicate that the average recovery for both
4.6. Storage Test
Tables 4.6.1. and 4.6.2. show the percent recoveries for
Storage Tests for
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 3 6 10 13 17 |
98.7 98.7 103.0 98.0 101.0 97.0 |
98.7 99.3 105.0 98.3 99.7 91.3 |
98.3 103.0 103.0 101.0 98.7 98.0 |
101.0 100.0 100.0 99.7 101.0 101.0 |
104.0 101.0 103.0 97.3 99.0 98.0 |
105.0 104.0 101.0 99.6 97.7 97.3 | |
|
| |||||||
Storage Tests for MDI (4.31 µg Spike)
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 3 6 10 13 17 |
102.0 97.0 102.0 99.1 98.6 95.3 |
102.0 95.1 99.1 97.2 98.8 93.7 |
101.0 101.0 101.0 101.0 96.8 99.5 |
102.0 99.3 101.0 99.3 103.0 101.0 |
105.0 99.3 101.0 96.5 97.9 100.0 |
109.0 99.1 101.0 100.0 97.9 99.5 | |
|
| |||||||
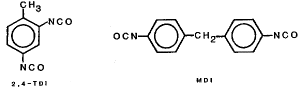
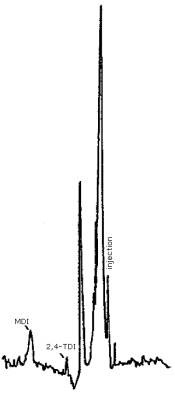
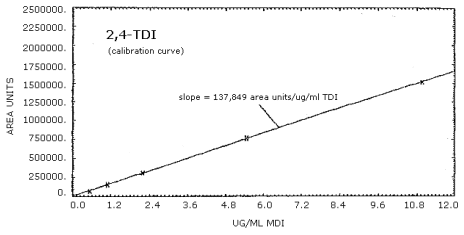
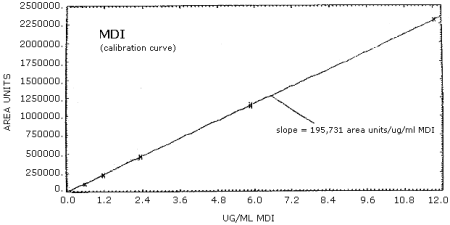
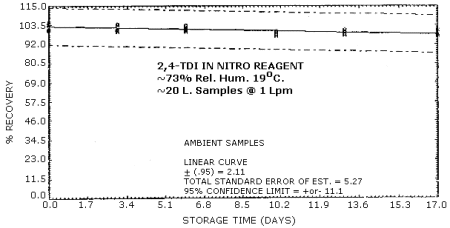
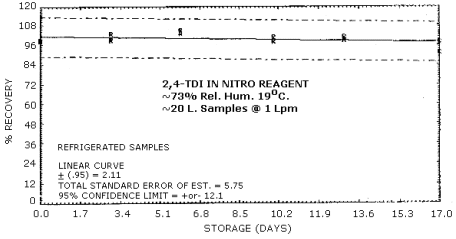
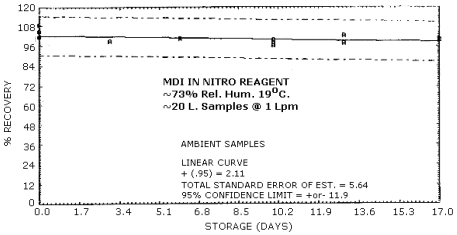
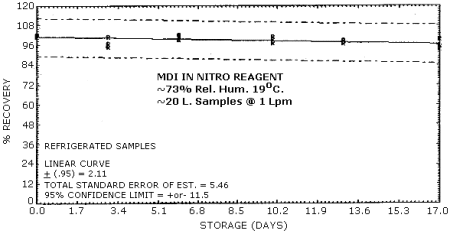
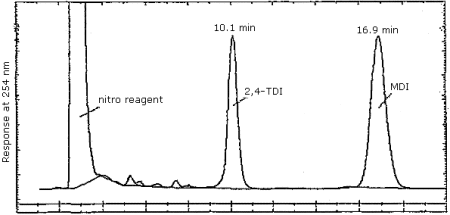
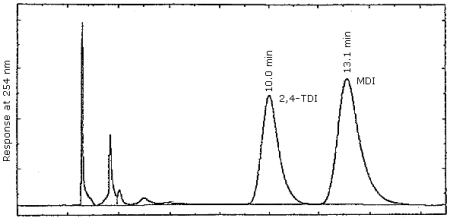
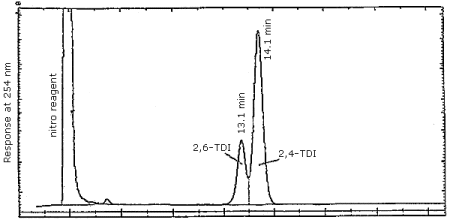
Figure 4.7.3. Reverse phase separation of 2,4- and 2,6-TDI
derivatives. Same conditions as in Section 3.5.1. except the mobile
phase is methanol/water/phosphoric acid 70/29.9/0.1 (v/v/v).
5. References
- 5.1. NIOSH Criteria Document On Diisocyanates, DHEW, (NIOSH)
Publication No.
5.2. NIOSH Criteria Document On Toluene Diisocyanate, HSM 73-11022, DHEW, Public Health Service, Center for Disease Control, NIOSH, 1973.
5.3. Dunlap, Sandridge and Keller, Anal. Chem., 48, 497, 1976.
5.4. "The Pros and Cons of Urethane Coatings", Mobay Chemical Corporation, Plastics and Coatings Division, Pittsburgh, PA 15205.
5.5. "Chemistry for Coatings", Mobay Chemical Corporation, Plastics and Coatings Division, Pittsburgh, PA 15205.
5.6. McFadyen, P., J. Chromatogr., 123, 468, 1976.
5.7. Spangnolo, Frank, J. Chromatogr. Sci., 14, 52, 1976.
5.8. The Merck Index, 9th Edition, Merck and Company, Rahway, NJ, 1976.
5.9. The Condensed Chemical Dictionary, 9th Edition, Hawley, Gessner, Van Nostrand Reinhold Co., N.Y., NY, 1977.
5.10. Telephone Conversation with Bob Sandridge, Mobay Chemical, New Martinsville, WV 28155, September, 1979.
5.11. "Urethane Polymers", V. 21, p. 64, Kirk-Othmer Encyclopedia of Chemical Technology, 2nd Edition, Interscience Publishers, 1970.
5.12. Vogt, C.R. Hastings, "Modification of an Analytical Procedure for Isocyanates to High Speed Liquid Chromatography", NIOSH Contract CDC-210-75-0052, University of Missouri, Columbia, MO, April, 1978.
5.13. Sango, J. Liq. Chromatogr., 2(6), 763, (1979).