(1,1-DICHLOROETHENE)
| Method no.: | 19 |
| Matrix: | Air |
| Target concentration: | 1 ppm (4.0 mg/m3) |
| Procedure: | Collection on coconut shell charcoal tubes, desorption with carbon disulfide and analysis by gas chromatography using an FID detector. |
| Recommended air volume and sampling rate: |
3 L at 0.2 L/min |
| Detection limit of overall procedure: (Section 4.1.) |
0.05 ppm (0.2 mg/m3) |
| Reliable quantitation limit: (Section 4.1.) |
0.05 ppm (0.2 mg/m3) |
| Standard error of estimate at the target concentration: (Figure 4.5.2.) |
7.0% |
| Special requirements: | Single analysis request. |
| Status of method: | Sampling and analytical method has been subjected to the established procedures of the Organic Methods Evaluation Branch. |
| Date: April 1980 | Chemist: Mike Shulsky |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
The first techniques of monitoring vinylidene chloride vapor were nonspecific total halide procedures. In the 1960s, area monitoring was introduced using infrared spectrophotometers. (Ref. 5.1.) In the mid 1970s, the charcoal tube collection method with gas chromatographic analysis was found to be applicable. (Ref. 5.2.)
1.1.2. Toxic Effects (This section is for information only and should not be taken as the basis for OSHA policy.)
Vinylidene chloride can effect several areas of the body: central nervous system, cardiovascular, skeletal, skin, respiratory, and liver or spleen. In addition, it has been shown to cause mutations in certain bacteria such as Escherichia coli. Only one study has been reported on possible carcinogenic effects in humans but it was reported that the limitations of this study do not permit an adequate evaluation. Vinylidene chloride can cause tumors in various organs of mice and rats. NIOSH recommends a 1 ppm exposure standard. (Ref. 5.3.)
1.1.3. Exposure
Vinylidene chloride is used throughout the plastics industry due to its ease of polymerization and copolymerization. Exposures can also occur in industries concerned with fabricated metal products, wholesale trade, leather products, chemicals, and special trade contractors. NIOSH estimates there are 58,000 workers potentially exposed to vinylidene chloride. (Ref. 5.3.)
1.1.4. Physical properties (Ref. 5.4. unless otherwise stated)
| molecular weight: | 96.94 |
| boiling point: | 31.7°C (760 mm) |
| specific gravity: | 1.2129 |
| flash point: | -15°C (open cup) |
| odor: | mild, sweet |
| color: | colorless |
| lower explosive limit: | 5.6% (Ref. 5.5.) |
| molecular formula: | H2C=CCl2 |
1.2. Limit defining parameters (All values are based on the recommended air volume.)
- 1.2.1. The detection limit of the analytical procedure is 0.6 ng
per 1-µL injection. This is the amount of vinylidene chloride which
gave a chromatographic peak large enough to distinguish it from the
leading edge of a contaminant peak present in the
CS2. (Section 4.1.1.)
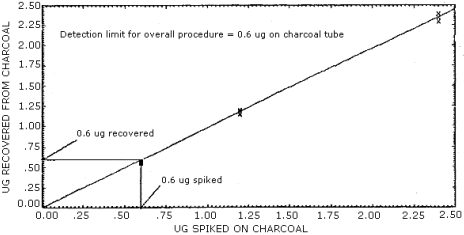
1.2.2. The detection limit of the overall procedure is 0.6 µg per sample (0.05 ppm or 0.2 mg/m3). This is the amount of vinylidene chloride spiked on a charcoal tube which will allow recovery of an amount equal to the detection limit of the analytical procedure. (Section 4.1.2.)
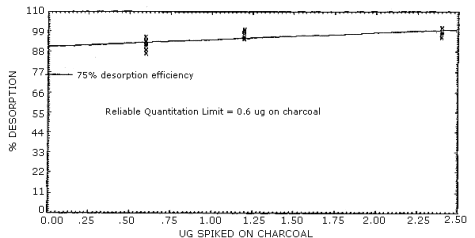
1.2.3. The reliable quantitation limit is 0.6 µg per sample (0.05 ppm or 0.2 mg/m3). This amount is equivalent to the detection limit of the analytical procedure. This is the smallest amount of analyte which can be quantitated within the requirements of a recovery of at least 75% and a precision (±1.96 SD) of ±25% or better. (Section 4.1.3.)
The reliable quantitation limit and detection limits reported in this method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of the analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Sensitivity
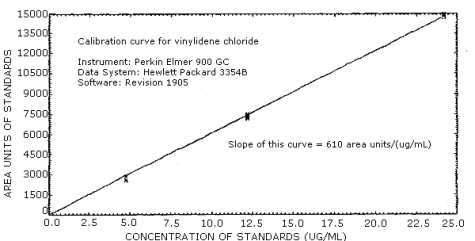
The sensitivity of the analytical procedure over a concentration range of approximately 0.5 to 2 times the target concentration, based on the recommended air volume, is 610 area units per µg/mL. This value is determined by the slope of the calibration curve. (Section 4.2.) The sensitivity will vary with the particular instrument and integration method used for the analysis.
1.2.5. Recovery
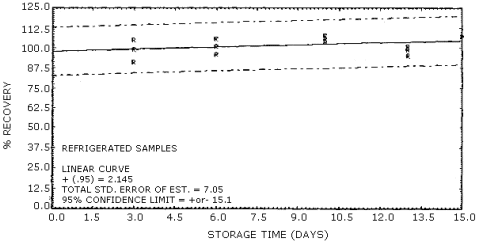
The recovery of vinylidene chloride averaged 95% over the concentration range of approximately 0.4 to 2 times the target concentration. (Section 4.4.) The recovery remained above 90% during the 15-day storage test. (Section 4.5.)
1.2.6. Precision (analytical method only)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.4, 1, and 2 times the target concentration is 0.032. (Section 4.1.4.)
1.2.7. Precision (overall procedure)
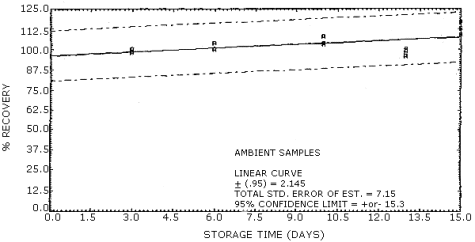
The overall procedure must provide results at the target concentration that are ±25% or better at the 95% confidence limit. The precision at this level for the 15-day storage test is ±15.3%. (Section 4.5.) This value includes an additional ±5% for sampling error.
1.3. Advantages
- 1.3.1. The sampling instruments are small and portable.
1.3.2. Multiple injections of each sample are possible.
1.3.3. Samples are stable for at least two weeks.
1.4. Disadvantages
- 1.4.1. The precision of the sampling pump is dependent on the
pressure drop across the charcoal tube.
1.4.2. The desorbing solvent, CS2, is toxic and must be handled cautiously.
1.4.3. High relative humidity decreases the capacity the of charcoal tube for vinylidene chloride vapors.
1.4.4. Charcoal will adsorb many organic vapors, so vapors present other than those requested for analysis should be listed as interferences.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. A personal sampling pump which can be calibrated within
±5%.
2.1.2. Charcoal tubes containing a 100-mg and 50-mg portion of coconut shell charcoal. For this evaluation, lot 107 charcoal tubes supplied by SKC, Inc. were used. The tubes were 7 cm long, with a 6-mm o.d., and a 4-mm i.d.
2.2. Reagents
None required
2.3. Sampling technique
- 2.3.1. Immediately before sampling, break open the ends of the
charcoal tubes. All tubes must be from the same lot of charcoal.
2.3.2. Connect the charcoal tube to the pump with a short piece of flexible tubing. The 50-mg portion of the charcoal tube is used as the backup section, therefore, it should be placed nearer the pump.
2.3.3. The exposed broken end of the tube should be shielded in some manner to protect the employee.
2.3.4. The tube should be placed vertically on the employee to avoid channeling through the charcoal.
2.3.5. Air being sampled should not pass through any hose or tubing before entering the charcoal tube.
2.3.6. Immediately after sampling, seal the ends of the tubes with the plastic caps.
2.3.7. With each set of samples, submit at least one blank charcoal tube from the same lot of charcoal as the samples. The blank tube should be treated in the same manner as the samples (break ends, seal, transport) except no air is drawn through it.
2.3.8. Transport the samples and corresponding paperwork to the lab for analysis.
2.3.9. If bulk samples are to be submitted for analysis, they should be sent in glass bottles sealed with Teflon-lined caps. Bulk samples must be sent in a completely separate container from the air samples.
2.4. Breakthrough
Studies to determine the 5% breakthrough value were done at approximately 1 ppm. Three determinations were performed.
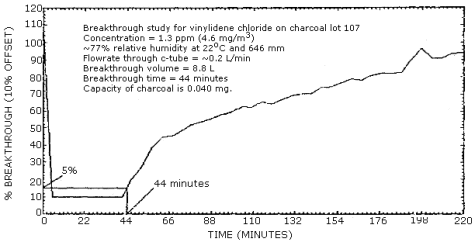
With the relative humidity at approximately 80% and the flow rate through the "A" portion of the tube at 0.2 L/min, the average breakthrough time was 45 min. The average air volume was 9.1 L and the average capacity was 41 µg. (Section 4.3.)
2.5. Desorption Efficiency
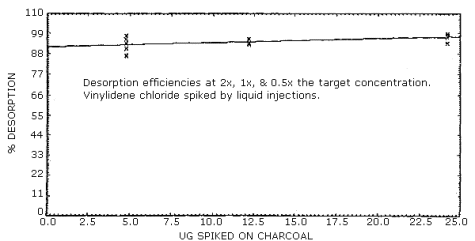
Desorption efficiencies were determined by liquid injections of CS2/vinylidene chloride solutions onto the 100-mg portion of charcoal tubes. Studies were done over the range of twice the target concentration down to the detection limit of the analytical procedure. Six tubes were spiked for each loading, (0.4, 1 and 2 times the target concentration) and the desorption efficiency averaged 95%. At lower loadings, the desorption efficiency remained in the same range (Section 4.4.).
2.6. Recommended air volume and sampling rate
A 3-L air volume is recommended at a flow rate not to exceed 0.2 L/min.
2.7. Interferences (Sampling)
- 2.7.1. High relative humidity will cause a decrease in the
capacity of the charcoal to adsorb vinylidene chloride. Since the
breakthrough and stability studies in this evaluation were done with
relative humidity at 70-80%, the recommended air volume has taken
this problem into account.
2.7.2. Charcoal will collect numerous other organic vapors. Any organic compound being used at or close to the sampling site should be listed as a possible interference.
2.8. Safety precautions (Sampling)
- 2.8.1. Eye protection should be worn while breaking the ends of
the charcoal tubes.
2.8.2. The exposed broken end of the charcoal tube should be shielded during sampling to protect the employee.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. Gas chromatograph equipped with a flame ionization
detector.
3.1.2. Microliter syringes; for example, 1-µL, 10-µL.
3.1.3. Analytical GC column: For this evaluation a 20-ft stainless steel column with 10% Tergitol on 80/100 Chromosorb P, AW DMCS was used.
3.1.4. Pipettes or dispenser for desorption of samples.
3.1.5. Volumetrics flasks; i.e., 5-mL, 10-mL.
3.1.6. A suitable method for peak area integration.
3.2. Reagents
- 3.2.1. Vinylidene chloride, reagent grade.
3.2.2. Carbon disulfide, gas chromatographic quality.
3.2.3. Internal standard solution, such as 0.04 µL of n-hexane per milliliter of CS2.
3.2.4. Helium, GC grade
3.2.5. Hydrogen, GC grade
3.2.6. Air, GC grade
3.3. Standard preparation
For this evaluation, an internal standard solution of 0.04 µL of n-hexane per milliliter of CS2 was used. Two separate stock standards were prepared at the concentration of 2 µL of vinylidene chloride per milliliter of internal standard. Analytical standards were made by diluting 10 µL of each stock standard to 5 mL with the internal standard solution. The resulting concentration of vinylidene chloride was 4.852 µg/mL. This concentration can be converted to an equivalent ppm by the following calculations.
Calculation for total µg in sample assuming 1-mL desorption volume:
(4.852 µg/mL)(1 mL) = 4.852 µg
Conversion to ppm (at 25°C and 760 mm Hg), assuming a 3-L air volume:
(0.004852 mg)(24.46)/[(0.003 m3)(96.94)] = 0.408 ppm
Since there is a 95% desorption efficiency, that correction can be made now.
Calculation: (0.408 ppm)/(0.95) = 0.43 ppm
From the integration method, the area for the vinylidene chloride peak from the above standard should be correlated to 0.43 ppm.
3.4. Sample preparation
- 3.4.1. The 100-mg portion of the charcoal tube is placed in one
vial while the 50-mg portion is placed in a separate vial. All glass
wool and urethane plugs are discarded.
3.4.2. Each vial is desorbed with 1 mL of internal standard solution and allowed to equilibrate for at least 20 min, with periodic shaking.
3.5. Analysis
- 3.5.1. Instrument conditions
| column: | 20-ft SS, 10% Tergitol on 80/100 Chromosorb P, AW DMCS. |
| oven: | 80°C |
| injector: | 150°C |
| detector: | 180°C |
| helium flow rate: | approx. 20 mL/min (carrier gas) |
| hydrogen flow rate: | 40 mL/min (detector gas) |
| air flow rate: | 250 mL/min (detector gas) |
3.5.2. A typical chromatogram appears in Figure 3.5.
3.6. Interferences
- 3.6.1. Any compound which has the same retention time as
vinylidene chloride or the internal standard would cause an
interference. By altering chromatographic conditions, the
interfering compound may be separated. Changing columns may also be
a viable solution, although the separation of vinylidene chloride
and CS2 is difficult or impossible on many
column packings.
3.6.2. If a sample is calculated to be above the target concentration a confirmation should be done by mass spectrometry or another suitable method. Retention time on one column is not considered proof of identity. Two different types of packing should be used to compare retention times. It is advisable to prepare standards at the same or approximate solution concentration as those samples which calculate to be above the target concentration.
3.7. Calculation
If the integration method is calibrated as described in Section 3.3.1. with the assumption of 3.0 L for the air volume, then the correction for the actual air volume is made by dividing the actual air volume by the 3.0-L assumed air volume and using this number to divide into the ppm value calculated from the integration method.
Example #1: Integration method gives a value of 1.0 ppm, but the actual air volume for the sample is 6.0 L.
| Actual Assumed |
6.0 L
3.0 L |
= 2.0 | 1.0 ppm
2.0 ppm |
= 0.50 ppm |
Example #2: Integration method gives a value of 1.0 ppm, but the actual air volume for the sample is 0.90 L.
| 0.90 L
3.0 L |
= 0.30 L | 1.0 ppm
0.30 ppm |
= 3.3 ppm |
3.8. Safety precautions
- 3.8.1. Eye protection should be worn at all times.
3.8.2. All solvents should be handled in an exhaust hood and away from any source of ignition, such as heated detectors or injectors.
4. Backup Data
- 4.1. Detection limit data
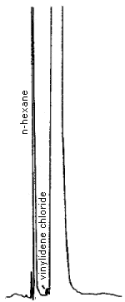
- 4.1.1. The detection limit of the analytical procedure of 0.6 ng
per injection was that amount of vinylidene chloride which gave a
peak large enough to distinguish it from the leading slope of the
contaminant peak from the CS2 which
followed it. (Figure 4.1.1.)
4.1.2. The detection limit of the overall procedure is 0.6 µg per sample (0.05 ppm or 0.2 mg/m3). (Figure 4.1.2.)
4.1.3. The reliable quantitation limit is 0.6 µg per sample (0.05 ppm or 0.2 mg/m3). This value is used since the desorption efficiency never fell below 75% and the precision was better than ±25%. (Figure 4.1.3.)
4.1.4. The precision of the analytical method at 0.4, 1, and 2 times the target concentration, given as a pooled coefficient of variation, is 0.032. Data are shown below.
Precision of the Analytical Procedure
|
| |||
| × target conc. µg/sample |
0.4× 4.8 |
1× 12.1 |
2× 24.2 |
|
| |||
| µg
recovered SD CV |
4.32 4.75 4.74 4.32 4.23 4.27 4.44 0.24 0.054 |
11.73 11.98 11.80 11.78 11.58 11.77 0.14 0.012 |
24.03 24.04 24.19 24.19 24.10 23.99 23.69 24.03 0.17 0.0071 |
|
| |||
4.2. Sensitivity
The sensitivity of this analytical procedure is defined as the area change per µg/mL as found from the slope of the calibration curve. For the instrument and data system used, the sensitivity was 610 area units per µg/mL. (Figure 4.2.)
4.3. Sampler capacity
The breakthrough studies were done at approximately 1 ppm, with a sampling rate of 0.2 L/min and a relative humidity of approximately 80%. Three determinations were done. The first determination is shown in Figure 4.3.
Sampler Capacity at 5% Breakthrough
|
| ||
| determination | air volume | capacity of charcoal |
|
| ||
| 1 2 3 |
8.8 L 9.5 L 9.1 L 9.1 L |
40 µg 42 µg 40 µg 41 µg |
|
| ||
4.4. Desorption efficiencies
Desorption efficiencies at 0.4, 1, and 2 times the target concentration, from samples prepared by liquid injection onto charcoal, averaged 95%. (Figure 4.4.) Desorption efficiencies at lower loadings of vinylidene chloride, down to the analytical detection limit, were also determined from samples prepared by liquid injection. These desorption efficiencies fell in the same range as those of the higher loadings. (Figure 4.1.3.)
Desorption Efficiency
|
| ||||||
| × target conc. µg/sample equivalent ppm |
0.6 0.05 |
1.2 0.1 |
2.4 0.2 |
0.4× 4.8 0.4 |
1× 12.1 1 |
2× 24.2 2 |
|
| ||||||
| desorption efficiency, % |
94 87 87 91 93 92 91 |
100 98 100 96 95 99 98 |
96 101 98 99 101 98 99 |
98 96 91 94 87 104 95 |
94 94 94 94 94 93 94 |
94 99 97 98 98 98 97 |
|
| ||||||
4.5. Storage tests
The storage tests were done by dynamically generating 36 samples at
approximately 1 ppm, and 80% relative humidity. Three liters of the
test atmosphere were drawn through each tube at 0.2 L/min. The first
six samples collected were analyzed on the same day but gave very low
recoveries compared to the remaining samples. By using the statistical
4d test (Ref. 5.6.), these first results were found to be out of the
allowed range, and dropped from the data table. Of the remaining 30
samples, 15 were stored at room temperature and 15 were stored at
Storage Tests
|
| |||||||
| storage time (days) |
%
recovery (refrigerated) |
%
recovery (ambient) | |||||
|
| |||||||
| 3 6 10 13 15 |
105 96 106 95 107 |
99 101 108 101 107 |
91 106 104 99 107 |
98 104 104 100 117 |
98 100 108 96 113 |
100 100 103 99 109 | |
|
| |||||||
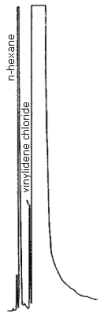
5. References
- 5.1. OH, M.G.; Fishbeck, W.A.; Townsend, J.C.; Schneider, E.J. "A
Health Study of Employees Exposed to Vinylidene Chloride." Journal
of Occupational Medicine, 18 (November, 1976) 735-738.
5.2. Severs, L.W.; Skory, L.K. "Monitoring Personnel Exposure to Vinyl Chloride, Vinylidene Chloride, and Methyl Chloride in an Industrial Work Environment." American Industrial Hygiene Association Journal, 36 (September, 1975) 669-676.
5.3. Bahlman, L.J.; Alexander, V.; Infante, P.F.; Wagoner, J.K.; Lane, J.M.; Bingham, E. "Vinyl Halides: Carcinogenicity." Current Intelligence Bulletin, No. 28, USDHEW/PHS/CDC/NIOSH/DOL/OSHA, Washington, D.C., Government Printing Office, 1979.
5.4. "Vinylidene Chloride." The Merck Index, 8th edition, Rahway: Merck and Company, Inc.
5.5. "Vinylidene Chloride." Dangerous Properties of Industrial Materials. 4th edition. New York: Van Nostrand Reinhold Company.
5.6. Skoog, D.A.; West, D.M. "Analytical Chemistry: An Introduction". New York, Holt, Rinehart, and Winston, Inc., p. 50.