| Method no.: | 20 |
| Matrix: | Air |
| OSHA standard: | 1 ppm (1.3 mg/m3)* (8-h TWA) |
| Target concentration: | 0.03 ppm* (0.04
mg/m3)(NIOSH recommended Ceiling for 120
min) *approximate values |
| Procedure: | Collection on sulfuric acid coated Gas Chrom R, desorption with water and analysis with a colorimetric screening or an HPLC procedure. |
| Recommended air volume and sampling rate: |
20 L at 0.1 to 1 L/min |
| Detection limit of the overall procedure: |
1.2 ppb (1.6 µg/m3) HPLC Procedure 5 ppb (6.5 µg/m3) Screening Procedure |
| Reliable quantitation limit: | 1.2 ppb (1.6 µg/m3) HPLC
Procedure 5 ppb (6.5 µg/m3) Screening Procedure |
| Standard error of estimate at the target concentration: (Section 4.6.) |
5.7% |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: September 1980 | Chemist: Carl J. Elskamp |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History of procedure
Hydrazine in air has been determined by collection in 0.1 M
hydrochloric acid and analysis by a colorimetric procedure using
In an attempt to utilize a more convenient sampling device and
make the analysis more specific, sulfuric acid-coated silica gel was
recommended for collection and a gas chromatographic procedure for
the
The U.S. Air Force School of Aerospace Medicine developed a field
procedure that involves collection on sulfuric acid coated Gas Chrom
R and colorimetric analysis using
A target concentration of 0.04 mg/m3 was chosen for the validation since animal studies had shown that hydrazine exhibits tumorigenic properties. (Section 1.1.2.) The sampling tube described in the Air Force method was chosen for validation tests. Since it does suffer from potential interferences, the colorimetric procedure was adapted for use as a screening test.
Hydrazine is very reactive so a derivatizing method was desired
for a more specific quantitative analytical procedure. No suitable
chromatographic procedure was found for the
The hydrazine derivative of benzaldehyde (benzalazine) was found to be an acceptable compound for HPLC analysis using a UV detector. Benzalazine has a maximum absorption at 300 nm. It was found in order to have a complete reaction, the pH had to be adjusted with a basic buffer and the samples heated in an 80°C water bath for one hour.
1.1.2. Toxic effects (This Section is for information only and should not be taken as the basis of OSHA Policy.)
- Hydrazine is a severe skin and mucous membrane irritant in
humans; in animals, it is also a convulsant and a carcinogen.
In humans, the vapor is immediately irritating to the nose and throat and causes dizziness and nausea; itching, burning, and swelling of the eyes develop over a period of several hours. Severe exposures of the eyes to the vapors causes temporary blindness lasting for about 24 hours. Recurrent exposure to hydrazine hydrate has been reported to cause contact dermatitis of the hands without systemic intoxication.
In humans, hydrazine is absorbed through the skin, by inhalation, and orally; systemic effects include weight loss, weakness, vomiting, excited behavior, and convulsions; the chief histologic findings are fatty degeneration of the liver and nephritis. (Ref. 5.6.)
NIOSH recommends an exposure limit of 0.04 mg/m3 determined as a ceiling concentration for 2 hours. The standard is designed to protect the safety of employees by "substantially reducing the risk of induced cancer and prevent other adverse effects, both acute and chronic. The recommended standard is based on the conclusion that valid evidence of skin absorption, blood and liver effects, and tumor induction in experimental animals is relevant to human exposure." (Ref. 5.7.)
The current OSHA standard of 1 ppm (1.3 mg/m3) is primarily based on Comstock's findings of symptoms or injury in dogs and rats exposed to 5 ppm. (Ref. 5.8.)
1.1.3. Exposure
"Hydrazine is used as a rocket propellant, polymerization catalyst, a blowing agent, a reducing agent, and oxygen scavenger in boiler water treatment, in the synthesis of maleic hydrazide, and in the manufacture of drugs. NIOSH estimates that approximately 9000 workers are potentially exposed to hydrazine in the United States." (Ref. 5.7.)
1.1.4. Physical properties (Ref. 5.7.)
| molecular formula: | H2NNH2 |
| CAS No.: | 000302012 |
| molecular weight: | 32.05 |
| description: | colorless, oily liquid, fuming in air. |
| odor: | penetrating odor resembling that of ammonia |
| density: | 1.0036 (25/4°C) |
| boiling point: | 113.5°C (at 760 mm Hg) |
| freezing point: | 1.4 - 1.5°C |
| explosive limits: | 4.7 - 100% by volume in air |
| flash point: | 38 - 52°C (open cup) |
| saturation concentration: | 18900 ppm |
| conversion factors: | 1 ppm = 1.31 mg/m3 |
| (at 760 mm and 25°C) | 1 mg/m3 = 0.76 ppm |
| solubility: | soluble in water, ethanol, and isobutanol; insoluble in chloroform and ether |
| vapor density: | 1.04 (air = 1) |
| vapor pressure: | 14.4 mm Hg at 25°C |
1.2. Limit defining parameters
- 1.2.1. Detection limit of the analytical procedure (HPLC
procedure)
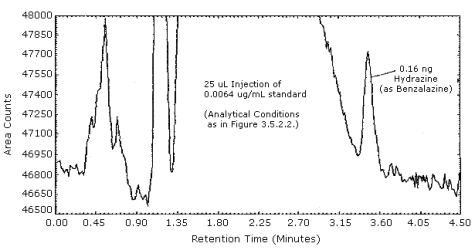
The detection limit of the analytical HPLC procedure is 0.16 ng per injection. This is the amount of hydrazine (analyzed as benzalazine) which will produce a peak which is approximately 5 times the baseline noise. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The overall detection limit of the colorimetric screening procedure is 0.13 µg of hydrazine per sample (0.005 ppm or 0.0065 mg/m3). This is the amount of hydrazine that will give a reading of 95% transmittance or 0.022 absorbance. (Section 4.4.)
The overall detection limit of the HPLC procedure is 0.032 µg of hydrazine per sample (0.0012 ppm or 0.0016 mg/m3). This is the amount of analyte spiked on the sampling device which allows recovery of an amount of analyte equivalent to the detection limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limits
Since the extraction efficiency is essentially 100%, the reliable quantitation limits are the same as the detection limits of the overall procedures for both screening and HPLC procedures.
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Sensitivity
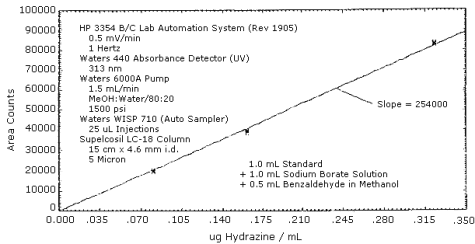
The sensitivity of the analytical HPLC procedure over a range representing 0.5 to 2 times the target concentration based on the recommended air volume is 254000 area units per µg/mL. The sensitivity is determined by the slope of the calibration curve. The sensitivity will vary somewhat with the particular instrument used in the analysis. (Section 4.3.)
1.2.5. Desorption efficiency
The recovery of analyte from the collection medium must be 75% or greater. The average recovery over the range of 0.5 to 2 times the target concentration is 99.8% as determined by the HPLC procedure. (Section 4.5.)
1.2.6. Precision (analytical method only)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1.0, and 2.0 times the target concentration is 0.008 by the HPLC procedure. (Section 4.3.)
1.2.7. Precision (overall procedure)
The overall procedure must provide results at the target
concentration that are ±25% or better at the 95% confidence level.
The precision at the 95% confidence level for the
1.3. Advantages
- 1.3.1. The sampling procedure is convenient.
1.3.2. Samples can be screened by the colorimetric procedure, thus, if no hydrazine is detected, the samples will not have to be analyzed by the more lengthy HPLC procedure.
1.3.3. The analytical procedure is sensitive and reproducible.
1.3.4. Reanalysis of samples is possible.
1.3.5. Samples are stable, even at room temperature.
1.4. Disadvantages
- 1.4.1. The amount of sample that can be taken is limited by the
total milligrams the acid coated Gas Chrom R will adsorb before
overloading.
1.4.2. The precision is limited by the reproducibility of the pressure drop across the tubes. The pumps are usually calibrated for one tube only.
1.4.3. As of this date, collection tubes are not commercially available, although SKC will be marketing them in the near future.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. An approved and calibrated personal sampling pump whose
flow can be determined within ±5%.
2.1.2. Acid coated Gas Chrom R collection tubes: The sampling tube should meet the specifications set forth in the Air Force method. (Ref. 5.5.) The tubes used for validation tests by the OSHA lab were prepared in the following manner: an amount of Gas Chrom R (30/60 mesh obtained from Applied Science Laboratories, Inc., P. O. Box 440, State College, PA 16801) was resieved through 30/60 mesh sieves. The fines were discarded. The remaining material in the 60 mesh sieve was washed repeatedly with deionized water, while still in the sieve, to remove remaining fines. The washed material was dried overnight at 110°C. A known amount of the dried Gas Chrom R was weighed into a round bottom flask. An amount of sulfuric acid equivalent to 20% of the weight of the Gas Chrom R is diluted with enough methyl alcohol so that when the solution is added to the Gas Chrom R, it will completely cover it. The flask is then fitted to a Rotovap and the solvent is stripped off, leaving the Gas Chrom R coated with sulfuric acid. Tubes were prepared by packing a one-inch section in the middle of a 4-in. fire-polished glass tube. The material was held in place with silanized glass wool.
2.2. Reagents
None required
2.3. Sampling technique
- 2.3.1. Connect the sampling tube to the sampling pump with
flexible tubing. Air being sampled should not pass through any hose
or tubing before entering the Gas Chrom R tube.
2.3.2. The tubes should be placed vertically during sampling.
2.3.3. Seal the sampling tubes with plastic caps immediately after sampling. Also, seal each sample with OSHA sealing tape lengthwise.
2.3.4. With each batch of samples, submit at least one blank tube from the same lot used for samples. This tube should be subjected to exactly the same handling as the samples (break, seal, transport) except that no air is drawn through it.
2.4. Breakthrough
No breakthrough data could be obtained since it was impossible to generate a known concentration of hydrazine in air. The Air Force reported no breakthrough for samples at concentrations between 0.04 and 27.0 mg/m3 collected at flow rates between 0.2 and 1.6 L/min with collection times up to 6.5 h. (Ref. 5.5.)
2.5. Desorption efficiency
The desorption efficiency from Gas Chrom R tubes spiked by liquid injections is essentially 100% from 0.5 to 2.0 times the target concentration for a 20-L air sample. Analysis was done by the HPLC procedure. (Section 4.5.)
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 20 L.
2.6.2. The recommended sampling rate is 0.1 to 1 L/min.
2.7. Interferences
- 2.7.1. It is unknown if any compound will severely interfere
with the collection of hydrazine on acid coated Gas Chrom R.
2.7.2. Suspected interferences should be listed on the sample data sheets.
2.8. Safety precautions
- 2.8.1. Attach the sampling equipment on the employee so that it
does not interfere with worker performance or safety.
2.8.2. Wear safety glasses at all times.
2.8.3. Follow all safety practices that apply to the work area where samples are being collected and avoid exposure to the analyte.
3. Analytical Procedures
- 3.1. Apparatus
- 3.1.1. Colorimetric screening procedure
- 3.1.1.1. Spectrophotometer capable of measuring at 465 nm.
3.1.1.2. A set of matched 1-in. cells.
3.1.1.3. Vortex mixer.
3.1.2. Analytical HPLC procedure
- 3.1.2.1. A liquid chromatograph equipped with a UV detector.
3.1.2.2. A column capable of separating benzalazine from benzaldehyde and any interferences. A reverse phase C18 was used for validation tests.
3.1.2.3. An electronic integrator or some other suitable method of measuring peak areas.
3.1.2.4. Small vials with Teflon-lined caps capable of holding 3 mL.
3.1.2.5. A 25-µL syringe for injections or an autosampler.
3.1.2.6. Water bath capable of heating to 80°C.
3.1.3. General
- 3.1.3.1. Screw cap (Teflon-lined) centrifuge tubes (or culture
tubes if can be used in a centrifuge) capable of holding a minimum
of 10 mL.
3.1.3.2. A rotator capable of holding the above tubes and rotating them approximately 60 times a minute.
3.1.3.3. A centrifuge capable of spinning at approximately 4000 RPM.
3.1.3.4. Volumetric flasks for preparing standards and making dilutions.
3.1.3.5. Pipets and syringes for preparing standards, making dilutions, and dispensing reagents.
3.2. Reagents
- 3.2.1. Colorimetric screening procedure
- 3.2.1.1. Glacial acetic acid - ACS grade.
3.2.1.2. Color reagent - 1.5 g of
3.2.2. Analytical HPLC procedure
- 3.2.2.1. 0.1 N Sodium borate,
Na2B4O7·10
H2O, ACS Grade.
3.2.2.2. Benzaldehyde solution- 1 mL reagent grade benzaldehyde to 100 mL with methyl alcohol.
3.2.2.3. HPLC grade methyl alcohol.
3.2.2.4. HPLC grade water.
3.2.3. General
- 3.2.3.1. Deionized water.
3.2.3.2. 0.1 N Sulfuric acid.
3.2.3.3. Hydrazine sulfate - ACS reagent grade.
3.3. Standard preparation
- 3.3.1. Dissolve 0.4060 g of hydrazine sulfate in 1 L of 0.1 N
sulfuric acid. This results in a concentration of 0.100 µg/µL.
3.3.2. Working standards can be prepared by diluting the above solution with 0.1 N sulfuric acid. Eight microliters of the 0.100 µg hydrazine/µL standard added to 5.0 mL of 0.1 N sulfuric acid is equivalent to 0.04 mg/m3 for a 20-L sample extracted with 5.0 mL water.
3.4. Sample preparation
- 3.4.1. General
- 3.4.1.1. Transfer the Gas Chrom R sections to separate
centrifuge (or culture) tubes.
3.4.1.2. Add 5.0 mL of deionized water to each tube.
3.4.1.3. Cap the tubes and rotate them for 20 min at approximately 60 rpm.
3.4.1.4. Centrifuge the tubes for 10 min at approximately 4000 rpm.
3.4.2. Colorimetric screening procedure
- 3.4.2.1. Transfer 3.0 mL of each sample and standard to
separate spectrophotometer cells.
3.4.2.2. Add 1.0 mL of color reagent
(
3.4.2.3. Mix the contents of each cell on a vortex mixer and allowed to stand for 10 min.
3.4.2.4. Add 10.0 mL of glacial acetic acid to each cell.
3.4.2.5. Mix again on the vortex and allow to stand for 5 min to allow any bubbles to dissipate.
3.4.2.6. Analyze samples at 465 nm as in section 3.5.
3.4.2.7. If hydrazine is detected, it will be necessary to quantitate it by the analytical HPLC procedure.
3.4.3. Analytical HPLC procedure
- 3.4.3.1. Transfer 1.0 mL of each sample and standard to
individual small vials.
3.4.3.2. Add 0.5 mL of benzaldehyde solution (benzaldehyde in methanol, 1 mL/100 mL) to each vial.
3.4.3.3. Shake the vials for a few seconds and then allow them to set for 5 min.
3.4.3.4. Add 1.0 mL of 0.1 N Sodium Borate to each vial and shake.
3.4.3.5. Heat the vials in the water bath at 80°C for 30 min.
3.4.3.6. Allow samples to cool to room temperature and analyze as in Section 3.5.
3.5. Analysis.
- 3.5.1. Colorimetric screening procedure
- 3.5.1.1. Samples and standards are read against a reagent
blank at 465 nm.
3.5.1.2. Absorbance is plotted versus µg of hydrazine per sample to construct a calibration curve.
3.5.1.3. If hydrazine is found to be present, it should be quantitated by the HPLC procedure.
3.5.1.4. The color is stable for at least 1 h.
3.5.2. Analytical HPLC procedure
- 3.5.2.1. HPLC parameters
UV detector at 313 nm
1.5 mL/min of
MeOH/H2O: 80/20
reverse phase
C18 column
25-µL direct injection of
samples from 3.4.3.6.
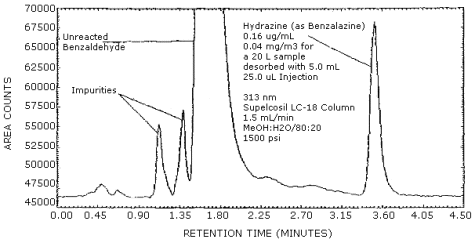
3.5.2.2. A chromatogram of a standard equivalent to 0.04 mg/m3 for a 20-L air sample is shown in Figure 3.5.2.2.
3.6. Interferences
- 3.6.1. Colorimetric screening procedure
- 3.6.1.1. Aromatic amines, methylhydrazine, and certain metal
ions are interferences. 1,1-Dimethylhydrazine is not an
interference, nor is ammonia below 300 ppm. (Ref. 5.5.)
3.6.1.2. If the samples are turbid, high absorbance readings will be obtained.
3.6.2. Analytical HPLC procedure
- 3.6.2.1. Any compound having the same retention time as
benzalazine and absorbing at the analytical wavelength is an
interference. HPLC parameters may be changed to circumvent these
interferences.
3.6.2.2. The benzaldehyde derivatives of methyl hydrazine, 1,1-dimethyl hydrazine, and phenyl hydrazine (benzaldehyde methylhydrazone, benzaldehyde dimethylhydrazone, and benzaldehyde phenyl hydrazone respectively) were found to elute earlier than the hydrazine derivative (benzalazine), so under normal circumstances would not interfere.
3.7. Calculations
- 3.7.1. Colorimetric screening procedure
- 3.7.1.1. The screening procedure is used only to report values
of none detected.
3.7.1.2. The amount of hydrazine found in the samples and blank is obtained from the calibration curve.
3.7.1.3. The calculated hydrazine concentration in air is found with the following equation:
mg/m3 = (A-B)/(C)
| where | A | = | micrograms of hydrazine per sample |
| B | = | micrograms of hydrazine per blank | |
| C | = | liters of air sampled |
3.7.2. Analytical HPLC procedure
- 3.7.2.1. The amount of hydrazine found in the samples and
blank is obtained from the calibration curve of peak area versus
micrograms of hydrazine per sample.
3.7.2.2. The calculated hydrazine concentration in air is derived from the following equation:
mg/m3 = (A-B)/(C)
| where | A | = | micrograms of hydrazine per sample |
| B | = | micrograms of hydrazine per blank | |
| C | = | liters of air sampled |
3.8. Safety precautions
- 3.8.1. Hydrazine is an animal carcinogen. Handle with due care.
3.8.2. All open chemicals should be used only in a fume hood.
3.8.3. Avoid any skin contact with all chemicals. Immediately flush contaminated areas with copious amounts of water.
3.8.4. Wear safety glasses at all times.
4. Backup Data
- 4.1. Detection limit of the analytical procedure
The detection limit of the HPLC procedure was determined by injecting 25 µL of a 0.0064 µg/mL standard. This is equal to 0.16 ng, or 0.0012 ppm (0.0016 mg/m3) for the recommended air volume of 20 L. The chromatogram is shown in Figure 4.1.
4.2. Detection limit of the overall procedure (HPLC procedure)
Two sample tubes were spiked with 8 µL of a 0.0040 µg/µL standard. The samples were analyzed the next day. The recoveries were 103.4 and 101.7%, or essentially 100% recovery. Therefore, the detection limit of the overall procedure is the same as the detection limit of the analytical procedure.
4.3. Sensitivity and precision (HPLC procedure)
The following data were used to determine the calibration curve and precision of the analytical HPLC method. The calibration curve is shown in Figure 4.3.
Sensitivity and Precision (HPLC Procedure)
|
| |||
| × target conc. µg/mL |
0.5× 0.0809 |
1× 0.1617 |
2× 0.3236 |
|
| |||
| area counts SD CV |
20136 19834 19714 19846 19569 19972 19828 227.1 0.0115 |
39513 39880 39707 39678 39354 39120 39542 273.6 0.0069 |
82818 82847 83327 82851 83052 83121 83003 201.5 0.0024 |
|
| |||
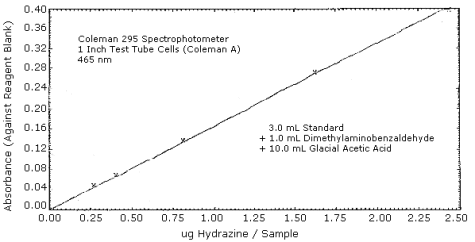
4.4. Sensitivity (colorimetric procedure)
The following data were used to construct a calibration curve for the colorimetric screening procedure. At 95% T (0.022 A) the detection limit is 0.13 µg/sample. The calibration curve is shown in Figure 4.4.
Sensitivity (colorimetric procedure)
|
| ||
| µg hydrazine/sample | % T | absorbance |
|
| ||
| 0.271 0.406 0.813 1.626 2.439 |
89.3 85.0 72.8 53.5 40.0 |
0.0491 0.0706 0.1379 0.2716 0.3979 |
|
| ||
4.5. Desorption efficiency
The desorption efficiency was determined by injection of aliquots of hydrazine standard onto Gas Chrom R tubes and desorbing with 5.0 mL of deionized water after standing overnight. Recoveries were done at 0.5, 1.0, and 2.0 times the target concentration for the recommended air volume by the HPLC method.
Desorption Efficiency
|
| |||
| × target conc. | 0.5× | 1× | 2× |
|
| |||
| desorption efficiency, % |
99.4 100.2 99.2 100.2 98.7 97.6 99.2 |
101.9 97.8 97.2 101.5 97.6 102.4 99.7 |
99.7 101.1 100.1 100.5 101.6 99.5 100.4 |
|
| |||
4.6. Storage data
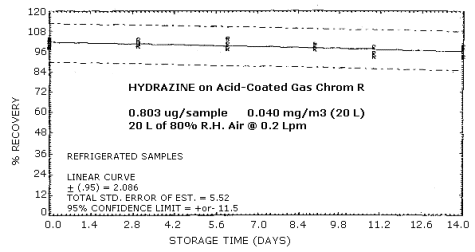
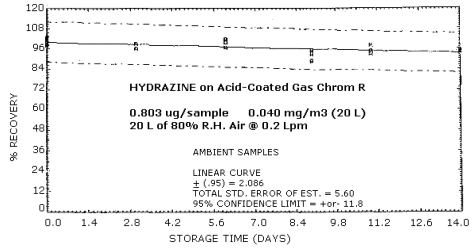
Samples were prepared by injecting 8.0 µL of a 0.10037 µg/µL standard onto acid coated Gas Chrom R tubes. Twenty liters of air at approximately 80% relative humidity were pulled through each sample at 0.2 L/min. Due to the restricted amount of time, the samples were analyzed by the colorimetric procedure. Six samples were analyzed immediately and fifteen samples were stored at refrigerated (approximately 0°C) and ambient temperatures (approximately 23°C). These stored samples were analyzed over a period of 14 days. The coefficient of variation for standards run during this study was 0.018. The results are shown graphically in Figures 4.6.1. and 4.6.2.
Storage Tests
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 0 3 6 9 11 14 |
99.7 103.3 99.3 97.5 97.3 93.1 93.1 |
101.5 98.8 102.8 103.7 99.1 94.9 99.0 |
102.4 98.3 100.6 -- 100.1 98.5 96.7 |
99.7 103.3 96.1 97.0 92.8 98.5 94.0 |
101.5 98.8 99.3 100.1 95.5 93.1 94.9 |
102.4 98.3 96.1 -- 89.1 94.5 93.6 | |
|
| |||||||
5. References
- 5.1. U.S. Department of Health, Education, and Welfare: Hydrazine.
Method No. S237. "NIOSH Manual of Analytical Methods", Vol. 3. 2nd ed.
Public Health Service, Center for Disease Control, National Institute
for Occupational Safety and Health. Cincinnati, Ohio (1977).
5.2. Audrieth, L.F.; Ogg, B.A. "The Chemistry of Hydrazine"; John Wiley and Sons, Inc.: New York, 1951; p. 164-65.
5.3. U.S. Department of Health, Education and Welfare: Hydrazine Compounds in Air. P & CAM #248. "NIOSH Manual of Analytical Methods": Vol. 1. 2nd ed. Public Health Service, Center for Disease Control, National Institute for Occupational Safety and Health. Cincinnati, Ohio (1977).
5.4. Cook, L.R., Glenn, R.E. and Podolak, G.E.; Am. Ind. Hyg. Assoc. J. 1979, 40, 69-74.
5.5. Department of the Air Force, USAF School of Aerospace Medicine (AFSC), "Hydrazine in Air", Brooks Air Force Base, Texas (1979).
5.6. Proctor, N.H.; Hughes, J.P. "Chemical Hazards of the Workplace"; J. B. Lippincott Company: Philadelphia, 1978; p. 284-285.
5.7. U.S. Department of Health, Education, and Welfare: "Criteria for a Recommended Standard. Occupational Exposure to Hydrazines", Public Health Service, Center for Disease Control, National Institute for Occupational Safety and Health, Cincinnati, Ohio (1978).
5.8. Sutton, W.L.: "Heterocyclic and Miscellaneous Nitrogen Compounds". In Fassett, D.W., and Irish, D.D. (eds.): "Toxicology" Vol. 2. In Patty, F.A. (ed.): "Industrial Hygiene and Toxicology", ed. 2., pp. 2218-2219, 2222-2225. New York: Interscience, 1963.