| Method no.: | 23 |
| Matrix: | Air |
| Target concentration: | 18.6 µg/m3 |
| Procedure: | Samples are collected by drawing a known volume of air through a midget, fritted bubbler containing isopropanol. Analysis is by high performance liquid chromatography using an ultraviolet detector. |
| Recommended air volume and sampling rate: |
250 L at 1 L/min |
| Reliable quantitation limit: | 3.5 µg/m3 |
| Standard error of estimate at the target concentration: (Section 4.7.) |
6.1% |
| Special requirements: | The samples must be protected from light during and after sampling. Ambient temperature storage tests indicate that recovery of the analyte falls below 75% about 6 days after collection (Section 4.7.). Therefore, samples must be either stored in a freezer or analyzed within 6 days after collection. It is strongly recommended that samples be refrigerated from the time they are collected until the time of analysis. |
| Status of method: | A sampling and analytical method that has been subjected to the established procedures of the Organic Methods Evaluation Branch. |
| Date: January 1981 | Chemist: Warren Hendricks |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
N-Nitrosodiphenylamine (NDFA) has been determined using thin layer (Ref. 5.1.), liquid (Ref. 5.2.), and gas (Ref. 5.3.) chromatographic techniques. Methods that utilize gas chromatography are somewhat tedious because a cleanup procedure, to separate NDFA from diphenylamine (DFA), must be performed prior to GC analysis. The cleanup procedure is necessary because NDFA decomposes completely at normal GC injection port temperatures to form DFA. This means that if DFA is present in the sample, it is an interference and unless it is removed, NDFA results will be high. (Ref. 5.3.)
NDFA is a fragile substance. The bond energy between the two nitrogen atoms is only 11 kcal/mole. This value is quite small when compared to the N-NO bond strength of dimethylnitrosamine which is 52 kcal/mole and the 70 to 90 kcal/mole bond dissociation energies of the C-N, C-C and C-H bonds in most organic molecules. (Ref. 5.4.)
The comparative instability of NDFA is demonstrated by the relative ease with which the agent can transfer its nitroso (NO) moiety to other amines. This process is known was transnitrosation and it can occur in dilute acid or organic solvents upon mild heating. (Ref. 5.5.) There are biological implications of transnitrosation which will be discussed in the toxicology section of this method.
NDFA has the ability to perform an interesting variation of
transnitrosation on itself. In dilute HCl, the N-nitroso group can
migrate to the para position on one of the phenyl rings resulting in
the formation of
The inherent instability of NDFA makes air sampling for the agent difficult. Airborne NDFA has been collected using Thermosorb-N cartridges, a commercial nitrosamine air sampling device produced by Thermo Electron Corp. of Waltham, Massachusetts. (Ref. 5.8.) Preliminary work performed in our laboratory indicates that there are serious stability problems after ambient temperature storage for NDFA spiked on Thermosorb-N cartridges. The recovery after only three days storage was less than 50% and it was for this reason that the Thermosorb-N sampling approach was not pursued.
Several nitrosamines have been collected using bubblers containing 1 N KOH. (Ref. 5.9.) The ambient temperature stability of NDFA in 1 N KOH was evaluated and it was found that after storage for 16 h about 75% of the amount spiked could be recovered. Only about 30% was recovered after 3 days of storage. Similar samples placed in a freezer gave NDFA recoveries near 100% after 25 days of storage.
Methods have been evaluated for collection of nitrosamines using pretreated Florisil tubes (Refs. 5.10. and 5.11). When this device was used in NDFA storage tests, low recovery was obtained after 5 days of storage at ambient temperatures. In each case the recovery was less than 50%. Refrigerated samples gave recoveries near 100%. When the stability test was repeated using NDFA spiked on untreated Florisil tubes, the recovery after 4 days of ambient temperature storage was about 85%. The results of the Florisil tube study indicate that the serious stability problems associated with pretreated Florisil tubes were a result of a reaction between the nitroso moiety of NDFA and the added nitrosation inhibitor. Perhaps a similar argument may be offered to help explain the poor NDFA stability encountered with Thermosorb-N cartridges.
In order to evaluate filters as a possible collection media for NDFA, samples were taken over an open bottle containing NDFA using 37-mm diameter glass fiber filters and also 37-mm diameter PTFE filters at about 1 L/min. The filters were contained in cassettes with cellulose back-up pads. Upon analysis, more NDFA was found on back-up pads than on the filter materials. The PTFE filter was particularly inefficient. The experiment was repeated with NDFA spiked on the filters. After 100 L of air at 80% relative humidity were drawn through the device only about 2% of the amount spiked was recovered from the filters and backup pads. In another experiment, untreated Florisil absorbent tubes were placed in series behind the filter cassettes and about 75% of the spiked NDFA was found on the Florisil tube after drawing 100 L of humid air through the system. Following these results, filters were abandoned as possible collection media for NDFA.
Because the storage stability of NDFA in isopropanol was similar to that obtained using untreated Florisil tubes and also because airborne NDFA may contain a particulate fraction that solid absorbents may not efficiently collect, isopropanol bubblers were evaluated as a sampling device for the agent.
Because of the thermal instability of NDFA, the obvious method to
separate the agent from potential interferences was high performance
liquid chromatography. Several detection systems were available
which included ultraviolet detectors, a photoconductivity detector
and the Thermal Energy Analyzer (TEA). The lowest detection limit
was obtained with the TEA but it does not respond to
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis for OSHA policy.)
NDFA has been shown to cause severe irritation (within 24 h) when 500 mg of the agent was applied to the eye of the rabbit. The oral LD50 was reported to be 1650 mg/kg for the rat and 3850 mg/kg for the mouse. The lowest published toxic dose, administered orally to the mouse over 78 weeks in discreet doses, was 410 g/kg and the toxic effects were neoplastic in nature. When 1000 mg/kg NDFA was given subcutaneously to the mouse, the toxic effects were, again, neoplastic. (Ref. 5.12.)
There is considerable disagreement in the literature regarding the carcinogenicity of NDFA. In an early study, published in 1961, NDFA had no carcinogenic activity. In that study, 1070 µg of NDFA suspended in 1 mL of 1% aqueous methylcellulose was given 5 times a week to rats by stomach tube. It has been shown that methylcellulose is not carcinogenic and it does not interfere with the carcinogenic activity of another compound. The treatment was discontinued after 45 weeks and the experiment was terminated after 53 weeks. Autopsy revealed that none of the 25 rats involved in the study had tumors. (Ref. 5.13.)
The results of the NDFA section of the well known Druckrey nitrosamine study, published in 1967, indicated that no tumors were observed following oral administration of 120 mg/kg NDFA to rats daily. The total dose was 65 g/kg. (Ref. 5.14.)
The lack of carcinogenic activity for NDFA has been explained using the following arguments:
- (1) Nitrosamines are thought to have to undergo biological
activation to form the ultimate carcinogen. The activation
involves the enzymatic hydroxylation of the carbon atom
immediately adjacent (in the alpha position) to the nitroso group.
The hydroxylation reaction requires that the alpha carbon possess
at least one hydrogen atom. Since NDFA is an aromatic substituted
nitrosoamine, it has no hydrogen on either of the alpha carbon
atoms and therefore, the compound cannot undergo enzymatic
activation.
(2) When the N-methyl or N-ethyl groups of highly carcinogenic N-nitrosamines are replaced with N-phenyl groups, carcinogenic activity may be lost because of steric hindrance.
(3) The strong carcinogenic activity of the dialkyl nitrosamines may be related to the high electron density about the nitroso group. When the N-alkyl groups are replaced with N-phenyl substituents, the phenyl rings may function as electron traps and redistribute the electron density about the nitroso moiety. (Refs. 5.13. and 5.14.)
NDFA is used as a retarder in the vulcanization of rubber. Since bladder cancer rates are higher among men employed in the rubber industry than in the general population, the agent's possible carcinogenicity has been further investigated. The results of a study, published in 1979, indicated that the agent is carcinogenic to rats but not mice. In this study, NDFA was mixed with food and given to the animals ad libitum for approximately 100 weeks. The doses were 4000 and 2000 ppm for rats of both sexes, 20,000 and 10,000 ppm for male mice and 10,000 and 5,000 ppm for female mice. The doses given to female mice were lowered to 4,000 and 1,000 ppm at the 38th week because of excessive weight loss. The time-weighted average doses for the female mice were 5741 and 2315 ppm. The administration of NDFA mixed in solid food produced a significant incidence of dose related urinary bladder tumors in rats of both sexes. No tumors were observed in mice of either sex at an incidence that was significantly higher in the dosed group than in the corresponding control group. However, urinary bladder inflammation occurred at high incidence and an abnormal increase in the epithelium of the bladder, at low incidence, was observed in the dosed groups of mice of both sexes. Neither lesion occurred in the corresponding control groups. (Refs. 5.15. and 5.16.)
The positive results of the Cardy experiment are in contrast to earlier negative work. It was not possible to accurately quantify the total dose given to rats in the Cardy study but the maximum daily intake of NDFA was estimated to be 320 mg/kg for female and 240 mg/kg for male rats. These doses, administered for 2 years, are higher than those given in the Druckrey experiment and may account for the difference in results. (Ref. 5.16.)
It is possible that the bladder tumors arose not from exposure to NDFA but from exposure to another nitrosamine which was formed in the rat's stomach. NDFA has been shown to be an effective transnitrosating species and the source of the other amine could have been the food. The food used in the experiment was not analyzed for nitrosatable amines and none is available for subsequent analysis. Therefore, the question of carcinogenicity was not resolved with certainty but this explanation could account for the difference in results between the experiments. The lack of effect in mice can be attributed to the well known resistance, in comparison to rats, of mice to carcinogenesis by nitrosamines. (Ref. 5.16.)
1.1.3. Operations where exposure occurs
NDFA is widely used as a vulcanization retarder in curing natural rubber and the synthetic elastomers: styrene-butadiene and nitrile-butadiene. 1.3 Million pounds of NDFA were produced by the United States in 1976. (Ref. 5.16.)
NDFA was found to be present in a human stomach after administration of a drink containing 100 mg of sodium bicarbonate, 300 mg of sodium nitrate, 1000 mg of glucose and 10 mg of diphenylamine. Nitrite, formed by bacterial reduction of nitrate in the stomach, enabled the synthesis of NDFA from diphenylamine. (Ref. 5.17.) This study is interesting in that it shows that exposure to NDFA can occur as a result of exposure to DFA and that in-vivo nitrosation of amines is possible in the absence of ingested nitrite.
1.1.4. Number of workers that face exposure - Unknown.
1.1.5. Physical properties (Refs. 5.12., 5.18. and 5.19.)
| CAS no.: | 86-30-6 |
| NCI no.: | C02880 |
| Synonyms: | diphenylnitrosamin (German), N-nitro- sodifenylamin (Czech), N-nitrosodi-phenylamine, diphenyl nitrosamine, Redax, Vulcatard, Vuklalent (Czech) |
| molecular structure: | Figure 1.1.5. |
| molecular weight: | 198.24 |
| physical appearance: | A yellow to brown or orange powder or flakes. |
| melting point: | 64-66°C |
| density: | 1.23 (at 20°C) |
| absorption maximum: | l = 290 nm, log e = 3.88 (in alcohol) |
| solubility: | Insoluble in water. Soluble in alcohol, acetone, benzene and ethylene dichloride. |
1.2. Limit defining parameters
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 1.4 ng of NDFA per injection. This is the amount of analyte which will give a peak whose height is about 5 times the amplitude of the baseline noise. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit for the overall procedure is 0.87 µg per sample (3.5 µg/m3). This is the amount of analyte spiked in the sampling device which allows recovery of an amount of analyte equivalent to the detection limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 0.87 µg per sample (3.5 µg/m3). This is the smallest amount of analyte which can be quantitated within the required 95% confidence limits of ±25%. (Section 4.3.)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Sensitivity
The sensitivity of the analytical procedure over a concentration range representing 0.5 to 2 times the target concentration is 16851 area units per µg/mL. The sensitivity is determined by the slope of the calibration curve. (Section 4.4.) The sensitivity will vary somewhat with the particular instrument used in the analysis.
1.2.5. Precision (analytical method only)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1, and 2 times the target concentration is 0.0408. (Section 4.6.)
1.2.6. Precision (overall procedure)
The 95% confidence interval for the 17-day storage test is ±12.7%. (Section 4.7.) This includes an additional ±5% for sampling error. The overall procedure must provide results at the target concentration that are ±25% or less at the 95% confidence level.
1.3. Advantages
- 1.3.1. The analytical procedure is quick, sensitive, and
reproducible.
1.3.2. Reanalysis of the samples is possible.
1.3.3. The analytical procedure permits the determination of two known decomposition products of the analyte. This determination is a measure of integrity of the sample. (Figure 4.8.)
1.4. Disadvantages
- 1.4.1. The use of bubblers is inconvenient.
1.4.2. Because of its flammable nature, the use of isopropanol in bubblers is potentially hazardous.
1.4.3. The samples must be refrigerated from the time they are collected until the time they are analyzed because they are not stable for storage at room temperature. (Section 4.7.)
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. An air sampling pump, the flow of which can be determined
to within ±5% at the 1 L/min recommended air flow rate with the
sampler in line.
2.1.2. Clean, dry 25-mL glass bubblers, fitted with matched ground joints and a fritted glass inlet.
2.1.3. Clean, dry 20-mL glass scintillation vials fitted with leakproof Polyseal caps or other suitable glass containers for transporting samples.
2.1.4. Glass pipets with rubber bulbs for transferring the isopropanol collection solution.
2.1.5. A means to refrigerate samples as soon as possible after collection. The use of dry ice and insulated containers is recommended.
2.2. Reagents
- 2.2.1. Isopropanol, HPLC grade.
2.2.2. Dry ice
2.3. Sampling technique
- 2.3.1. Place approximately 15 mL of HPLC grade isopropanol in a
clean, dry glass bubbler prior to sampling. Connect the bubbler to
the sampling pump with flexible tubing. Place the bubbler in an
upright position. Do not allow sampled air to pass through any hose
or tubing before entering the bubbler. Because light will decompose
NDFA, wrap the bubbler with tape or other suitable material.
2.3.2. It will probably be necessary to interrupt sampling to add more HPLC grade isopropanol to the bubbler because isopropanol evaporates at the rate of 6 to 7 mL/h when sampling air (30°C) at 1 L/min. It has been shown that collected NDFA will not be lost in evaporated isopropanol. (Section 2.4.)
2.3.3. After sampling, the isopropanol is transferred to a glass vial for shipping. Rinse the inlet tube and bubbler assembly with several 1-mL portions of isopropanol and transfer the washes to the vial containing the sample.
2.3.4. Insure that the vial containing the sample is leakproof and then wrap each sample end to end with official OSHA seals. Protect the vial from light.
2.3.5. Because NDFA is not stable for storage at room temperature, (Section 4.7.) samples must be refrigerated with dry ice immediately after collection.
2.3.6. Samples must be transported to the laboratory packed in dry ice using an insulated container. Inform laboratory personnel of the impending arrival of the samples.
2.3.7. Indicate, in a conspicuous manner, on the shipping container that the contents must be placed in a freezer immediately upon arrival at the laboratory.
2.3.8. With each batch of samples, submit at least one blank sample. The blank should be subjected to the same handling as the samples except that no air is drawn through it.
2.3.9. If bulk samples are submitted for analysis, they should be shipped in sealed glass vials. Bulk samples should be refrigerated and protected from exposure to light.
2.3.10. List possible interferences on the sample data sheet.
2.4. Breakthrough
- 2.4.1. Retention efficiency
Two bubblers, each containing 15 mL of isopropanol, were placed in series and then 182 µg NDFA was added to the first bubbler. Air, at about 80% relative humidity and 22°C, was drawn through the device at 1 L/min. The volume of isopropanol in each bubbler was maintained at 10 to 15 mL by adding pure HPLC grade isopropanol as required. After 300 L of air had been sampled, about 4% of the NDFA added to the first bubbler was found in the second bubbler. About 94% of the NDFA added to the first bubbler was recovered from the sampling train. These results indicate that NDFA is stable, at least temporarily, once collected.
2.4.2. Vapor trapping efficiency
The recommended sampling device appeared to trap vaporous NDFA efficiently when samples were taken over an open container of NDFA and also when vapors were generated by butting a short piece of silanized glass tubing, containing a silanized glass wool plug spiked with NDFA, to the inlet of the bubbler and then drawing air through the device at 1 L/min.
The analysis of NDFA vapor samples indicated that nearly as much DFA as NDFA was collected. Since it was shown that NDFA is stable, at least temporarily, once collected and because the NDFA used in the experiment contained only a very small amount of DFA, it seems reasonable that the change from the solid to the vapor state is sufficient to decompose the agent. A NDFA decomposition product, the nitrosyl radical (·NO), is a stable gas (Ref. 5.4.) and it is available to react with any nitrosatable amine. If DFA is encountered when sampling for NDFA and if DFA is not used in the process then NDFA may have decomposed and other nitrosamines may have formed. It is prudent to sample for other nitrosamines when sampling for NDFA.
2.5. Recommended air volume and sampling rate
- 2.5.1. The recommended air volume is 250 L.
2.5.2. The recommended sampling rate is 1 L/min.
2.6. Interferences (sampling)
- 2.6.1. Since it is possible that precursors of NDFA: DFA,
tertiary amines with the
In an experiment to determine if the artifactual formation of NDFA was significant, 2 mg of DFA were placed in a bubbler containing 15 mL of isopropanol. One hundred-eighty liters of air containing 6 ppm NOx as NO2 (2 mg total) were sampled at 1 L/min and the solution was analyzed for NDFA. About 2% NDFA (based on the available DFA) was formed but only about 70% by weight of the added DFA was recovered. DFA has been used as an indicator in oxidization/reduction titrations (Ref. 5.20.) and in spot tests for nitrite, nitrate, nitrous acid, and various other oxidizing agents. The product from DFA was N,N'-diphenylbenzidine. (Ref. 5.21.) It may be that the missing DFA was lost as a result of this reaction.
Since there are competing reactions and because the conditions under which the samples are taken and stored are not favorable for the production of NDFA, the artifactual formation of NDFA is not considered to be significant. In addition to this, unlike most other nitrosamines, NDFA is used in industry and its presence or absence can be predicted.
2.6.2. It is unknown if there are other interferences with the collection of NDFA in isopropanol bubblers.
2.7. Safety precautions (sampling)
- 2.7.1. Attach the sampling equipment to the worker in such a
manner that it will not interfere with work performance or safety.
2.7.2. Care must be exercised when sampling with isopropanol because it is a flammable solvent. Do not sample around ignition sources.
2.7.3. Follow all safety practices that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. High performance liquid chromatograph, equipped with
pump, sample injector, UV detector, chart recorder and necessary
hardware.
3.1.2. HPLC analytical column capable of separating NDFA, DFA,
and
3.1.3. An electronic integrator, or other suitable method to measure detector response.
3.1.4. Microliter syringes for sample injections.
3.1.5. Volumetric glassware for sample and analytical standard preparations.
3.1.6. Analytical balance.
3.2. Reagents
- 3.2.1. Isopropanol, methanol and water: HPLC grade.
3.2.2. N-Nitrosodiphenylamine, analytical standard quality.
3.3. Standard preparation
- 3.3.1. Stock standards are prepared by diluting a weighed amount
of NDFA with isopropanol. Remember that the agent is unstable and
that suitable precautions must be taken to insure the integrity of
the standards. It is recommended that the standards be stored in
dark bottles in a freezer. Old standards should be checked against
new standards often and dilution from stock standards to the working
range should be made daily. The unexpected appearance of a DFA peak
(Figure 4.8.) is one indication that the standard has decomposed. Do
not prepare mixtures of NDFA and DFA.
3.3.2. A solution containing 0.31 µg/mL NDFA in isopropanol is equivalent to a NDFA air concentration of 18.6 µg/m3 providing that 250 L of that atmosphere is sampled with a bubbler containing 15 mL (final volume) of isopropanol.
3.4. Sample preparation
- 3.4.1. Make sure that the samples are stored in a freezer and
are protected from light as much as possible.
3.4.2. Measure the volume of isopropanol in the sample transport vial with a graduated cylinder, to the nearest 0.1 mL.
3.5. Analysis
- 3.5.1. HPLC conditions
| column: | DuPont Zorbax CN (4.6 mm × 25 cm) |
| mobile phase: | methanol/water - 60/40 (v/v) |
| flow rate: | 1 mL/min |
| UV detector: | 280 nm (fixed wavelength) |
| injection Volume: | 25 µL |
| retention time: | 6 min |
3.5.2. If a dual wavelength detector, such as the Waters
Associates Model 440, is used, also monitor the response at 405 nm
because this is near the UV absorption maximum for
3.5.3. Chromatogram (Backup Data Section, Figures 4.5. and 4.8.)
3.5.4. Detector response is measured with an electronic integrator or other suitable means.
3.5.5. An external standard procedure is used to prepare a calibration curve using at least 3 different standard solutions. The calibration curve is prepared daily. The integrator is calibrated to report results in µg/mL.
3.5.6. Bracket the samples with analytical standards.
3.6. Interferences (analytical)
- 3.6.1. Any compound that absorbs UV light at 280 nm and has the
same retention time as NDFA is an interference.
3.6.2. Potential interferences include DFA and
3.6.3. HPLC parameters may be changed to circumvent most other interferences.
3.6.4. Retention time on a single HPLC column is not proof of chemical identity. Samples should be confirmed by an independent method when required. NDFA is not amenable to gas chromatographic procedures. One possible confirmatory procedure includes use of the recommended analytical procedure with a Tracor Model 965 photoconductivity detector (use the mercury lamp) in series after the UV detector. The possible interference is not separated from NDFA but it is highly unlikely that two different compounds will give the same response to two such dissimilar detection systems. Another possible confirmatory procedure recommends use of the TEA and separation by liquid chromatography using a silica gel analytical column. The mobile phase was 99/l (v/v) isooctane/acetone solution.
3.7. Calculations
- 3.7.1. The integrator values in µg/mL are used for reference
only. More reliable results are obtained by use of a calibration
curve. The detector response, for each standard, is compared to its
concentration in µg/mL and the best straight line through the data
points is determined by linear regression.
3.7.2. The concentration, in µg/mL, for a particular sample is determined by comparing its detector response to the calibration curve.
3.7.3. The air concentration for a sample result is calculated by the following equation:
NDFA, µg/m3 = (A)(B)/C
| where | A | = | µg/mL from Section 3.7.2. |
| B | = | volume (mL) of isopropanol from Section 3.4.2. | |
| C | = | air volume (m3) |
3.8. Safety precautions (analytical)
- 3.8.1. Sample and standard preparations should be done in a fume
hood. Avoid exposure to both standards and samples.
3.8.2. Avoid skin contact with the solvents.
3.8.3. Confine the use of solvents to a fume hood.
3.8.4. Wear safety glasses in all laboratory areas.
3.8.5. NDFA and
4. Backup Data
- 4.1. Detection limit of the analytical procedure
The detection limit for NDFA was 1.4 ng (25 µL × 0.058 µg/mL) per injection. This amount of analyte gave a peak whose height was about 5 times the amplitude of the baseline noise. (Figure 4.1.)
4.2. Detection limit of the overall procedure
The detection limit of the overall procedure was 0.87 µg (15 mL × 0.058 µg/mL) per sample.
4.3. Reliable quantitation limit
The reliable quantitation limit was the same as the detection limit of the overall procedure since the interval about the detection limit was less than ±25% at the 95% confidence level. This was determined by replicate injections from a standard solution.
Reliable Quantitation Limit Data
|
| |||
| peak height, mm* | statistics | ||
|
| |||
| 10.1 | |||
| 11.0 | = | 10.76 | |
| 10.5 | SD | = | 0.4505 |
| 11.2 | CV | = | 4.187% |
| 11.0 | ±1.96(CV) | = | ± 8.2% |
|
| |||
| * Response was measured as peak height because integrator peak areas were unreliable at this low concentration. | |||
4.4. Sensitivity
A calibration curve for NDFA is shown in Figure 4.4. The slope of the regression line is a measure of the sensitivity of the method.
4.5. Chromatogram
A typical chromatogram for NDFA is presented in Figure 4.5.
4.6. Precision of the analytical method
These data represent multiple injections from standard solutions. The injection volume was 25 µL and the concentrations of the standards were 0.16, 0.31, and 0.62 µg/mL.
Precision of the Analytical Method
|
| |||
| × target conc. | 0.5× | 1× | 2× |
| µg/mL | 0.16 | 0.31 | 0.62 |
|
| |||
| µg/mL found | 0.158 | 0.307 | 0.605 |
| 0.174 | 0.301 | 0.590 | |
| 0.149 | 0.302 | 0.610 | |
| 0.167 | 0.302 | 0.647 | |
| 0.164 | 0.314 | 0.640 | |
| 0.174 | 0.295 | 0.637 | |
| 0.173 | 0.292 | 0.630 | |
| 0.1656 | 0.3019 | 0.6227 | |
| SD | 0.009432 | 0.007290 | 0.02118 |
| CV | 0.05696 | 0.02415 | 0.03401 |
|
| |||
4.7. Storage
- 4.7.1. The data in Table 4.7.1. represent the results of storage
tests conducted at ambient (20 to 25°C) and reduced (-5°C)
temperatures. The samples were prepared by placing 15-mL aliquots of
a solution containing 0.31 µg/mL NDFA in isopropanol into 20-mL
glass scintillation vials. Three separate vials were analyzed on the
day indicated. The data in Table 4.7.1. are presented graphically in
Figures 4.7.1. and 4.7.2.
Storage Tests
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (ambient) | (refrigerated) | |||||
|
| |||||||
| 0 | 102.6 | 105.5 | 98.4 | 103.9 | 99.0 | 90.3 | |
| 3 | 85.2 | 87.8 | 85.0 | 95.6 | 98.6 | 96.5 | |
| 6 | 79.1 | 75.7 | 77.9 | 98.1 | 93.4 | 97.8 | |
| 9 | 67.6 | 68.2 | 63.3 | 98.3 | 101.2 | 100.6 | |
| 13 | 59.1 | 64.3 | 68.4 | 98.2 | 97.8 | 101.5 | |
| 17 | 56.6 | 68.0 | 61.4 | 101.9 | 104.4 | 99.0 | |
|
| |||||||
4.7.2. The early part of the ambient temperature storage test
gave the expected linear appearance/disappearance relationship
between DFA and NDFA, but as the test progressed, it became obvious
that some competing mechanism was removing DFA from solution. No
determination for the rearrangement product
Recovery of Samples Stored at Ambient Temperature
|
| ||||||
| days of storage | 3 | 6 | 9 | 13 | 17 | 42 |
|
| ||||||
| % recovery (corrected for DFA) | 97 | 97 | 88 | 90 | 92 | 84 |
|
| ||||||
Results for days 3 to 17 are the average of three samples. Day 42 was a single sample.
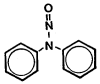

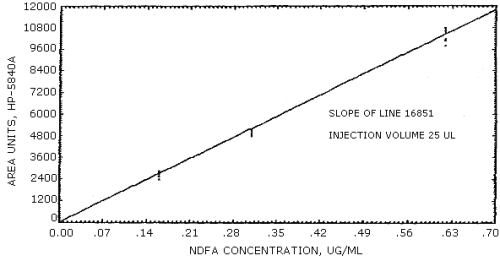

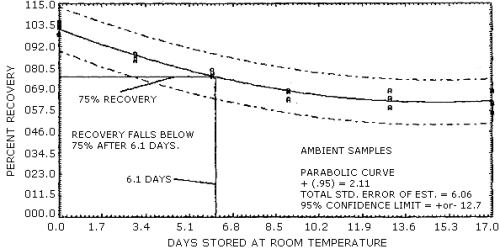
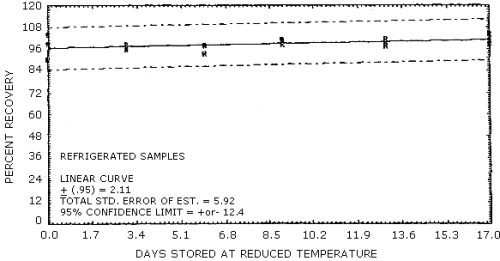
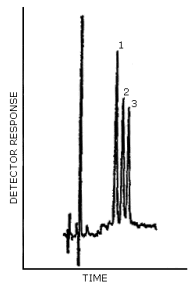
Figure 4.8. Chromatogram for a mixture containing
5. References
- 5.1. Archer, A.W. J. Chromatogr., 108 401-404 (1975).
5.2. Vodicka, L., Kriz, J., Burda, J. and Novak, P. J. Chromatogr., 148 247-254 (1978).
5.3. Nitrosamines - Method 607 Federal Register 44(233) 69496-69500 (Monday, 12-3-79).
5.4. Instruction Manual - Thermal Energy Analyzer, Model TEA 502/LC, Thermal Electron Corporation, Waltham, Massachusetts 02154 3-3 (12-77).
5.5. Buglass, A.J., Challis, B.C. and Osborne, M.R. "IARC Scientific Publication No. 9" Bogovski, P., and Walker, E. A. Eds., 94-100 (1974).
5.6. March, J. "Advanced Organic Chemistry: Reactions, Mechanisms, and Structure" McGraw-Hill, Inc.: New York 430-431 (1968).
5.7. "Bioassay of p-Nitrosodiphenylamine for Possible Carcinogenicity", NCI: Bethesda, Report (DHEW/PUB/NIH-79-1746-NCI-CG-TR-190) (1978).
5.8. Interim Report #1, Health Hazard Evaluation Project No. HE79-109, Kelly-Springfield Tire Company, Cumberland, MD, USDHEW, CDC, NIOSH: Cincinnati (9-19-79).
5.9. Fajen, J.M., Carson, G.A., Roundbehler, D.P., Fan, T.Y., Vita, R., Goff, V.E., Wolf, M.H., Edwards, G.S., Fine, D.H., Reinhold, V., and Biemann, K. Science 205 1262-1264 (1979).
5.10. Hendricks, W. Diethylnitrosamine (Method 13, Organic Methods Evaluation Branch, OSHA Analytical Laboratory, Salt Lake City, Utah) Unpublished (8-79).
5.11. Hendricks, W. N-Nitrosomorpholine (Method 17, Organic Methods Evaluation Branch, OSHA Analytical Laboratory, Salt Lake City, Utah) Unpublished (1-80).
5.12. "Registry of Toxic Effects of Chemical Substances", 1978 Edition. (Lewis, R.J. and Tatken, R.L., Eds.) U.S. Department of Health, Education and Welfare, Public Health Service, Center for Disease Control, National Institute for Occupational Safety and Health, U.S. Government Printing Office, Washington, D.C. (1978).
5.13. Argus, M.F. and Hoch-Ligeti, C. J. Natl. Cancer Inst., 27 695-701 (1961).
5.14. Druckrey, D., Preussmann, R., Ivankovic, S. and Schmahl, D. Z. Krebsforsch 69(103) 103-201 (1967).
5.15. Cardy, R.H., Lijinsky, W., and Hilderbranat, P.K. Ecotoxicology and Environ. Safety 3, 29-35 (1979).
5.16. Bioassay, of N-Nitrosodiphenylamine for Possible Carcinogenicity NCI: Bethesda, Report (DHEW/PUB/NIH, 79-1720-NCI-CG-TR-164) (1979).
5.17. Sander, J., and Seif, F. Arzneim Forsch, 19(7) 1091-1093 (1969).
5.18. "The Condensed Chemical Dictionary", 9th Ed., Hawley, G.G., Van Nostrand Reinhold Co.: New York 619 (1977).
5.19. "CRC Handbook of Chemistry and Physics", CRC Press: Boca Raton, FL A892 (1979).
5.20. Tredwell, F.P. "Analytical Chemistry, Vol. II." John Wiley and Sons, Inc.: New York, 639(1948).
5.21. Welcher, F.W. "Organic Analytical Reagents: Vol. II." D. Van Nostrand Company, Inc.: New York (1947).