| Method no.: | 32 |
| Matrix: | Air |
| Target concentration: (PEL) |
19 mg/m3 (5 ppm) for
phenol 22 mg/m3 (5 ppm) for cresol (all isomers) |
| Procedure: | The analytes are collected on an |
| Recommended air volume and sampling rate: |
24 L and 0.1 L/min |
| Reliable quantitation limit: | 0.041 mg/m3 (0.01 ppm)
phenol 0.046 mg/m3 (0.01 ppm) cresol |
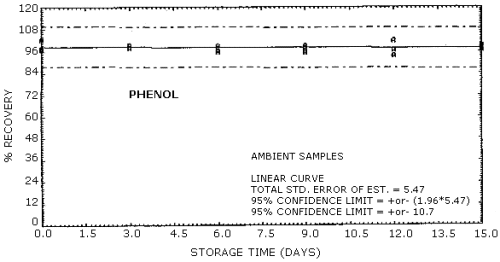
| Standard error of estimate for ambient storage samples: (Figures 4.8.1., 4.8.3.) |
5.47% for phenol 5.41% for cresol |
| Status of method: | A sampling and analytical method which has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: November 1981 | Chemist: Kevin Cummins |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
The analysis of phenol and cresol, like many chemicals in use for a long period of time, has evolved from a number of nonspecific colorimetric methods to more selective separation techniques using gas chromatography (GC) or high performance liquid chromatography (HPLC) (Refs. 5.1.5.3.). The analytical procedure presented in this method uses reverse phase HPLC with ultraviolet (UV) detection at 218 nm, since the unresolved cresol isomers respond equally at this wavelength. An alternate gas chromatographic method using flame ionization detection is also quite satisfactory. Although the GC method is less sensitive than the liquid chromatographic method, it does provide better resolution of the cresol isomers.
Air sampling and analytical methods for phenol and cresol
developed by NIOSH have been in use for several years. The NIOSH
phenol method uses an aqueous bubbler to collect vapors, whereas
cresol vapors are collected on a silica gel tube. Both of these
methods utilize gas chromatography with flame ionization detection
for analysis (Refs. 5.3. and 5.4.). Recently a very sensitive method
for detecting phenol in air has been developed by Kuwata, et al.
(Ref. 5.5.). This method uses a 0.1 N NaOH bubbler solution to
collect the phenol vapors followed by derivatization with
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy)
A number of cases of overexposure to phenol or cresol are
reported in the literature. Both compounds are rapidly absorbed
through the skin and can cause skin and eye burns upon contact.
Comas, convulsions, cyanosis and death can result from overexposure
to either compound. The ingestion of 15 g of phenol produced death
in a 19 year old woman within 20 h. Internally, cresol and phenol
affect the liver, kidneys, lungs, and vascular system. There is some
indication that cresol may be more toxic than phenol when inhaled.
Respiratory irritation in 8 of 10 human subjects exposed to 6
mg/m3 of
1.1.3. Workplace exposure
Phenol is used to make phenolic resins, caprolactam, bisphenol A and alkyl phenols. In 1972, 1.23 million tons of phenol were produced in the U.S. primarily from synthetic processes. An estimated 10,000 employees are potentially exposed to phenol. This does not include possible worker exposure to products containing phenol (Ref. 5.6.).
The majority of the cresols are derived from petroleum or coal
tar acids. In 1975, 151 million tons of cresol and cresylic acids
were produced in the U.S. Cresol is used to make phenolic resins,
tricresyl phosphate, disinfectants, and antioxidants.
1.1.4. Physical properties (Refs. 5.6. and 5.7.)
| phenol | |
| molecular weight: | 94.11 |
| melting point: | 40 - 41°C |
| boiling point: | 181.75°C |
| vapor pressure: | 0.35 mm Hg (25°C) |
| specific gravity: | 1.071 (25°C) |
| flash point: | 85°C (open cup) 79°C (closed cup) |
| odor threshold: | 3.8 mg/m3 |
| Soluble in water, ether, alcohol and benzene. Colorless to light pink solid. | |
| cresol (ortho-, meta-, and para-isomers) | |||
| m-cresol | |||
| MW: | 108.13 | 108.13 | 108.13 |
| mp: | 30.9°C | 12.0°C | 34.8°C |
| bp: | 191.0°C | 202.7°C | 201.9°C |
| vp(25°C): | 0.25 mm Hg | 0.15 mm Hg | 0.11 mm Hg |
| sp gr(20°C): | 1.048 | 1.034 | 1.35 |
| flash p: | 81.1°C | 86.1°C | 86.1°C |
| (closed cup) | |||
| odor | |||
| threshold: | 0.0028 mg/m3 | 0.034 mg/m3 | 0.0021 mg/m3 |
| Soluble in water, alcohol, ether, pet. ether and benzene. | |||
1.2. Limit defining parameters (The analyte air concentrations listed throughout this method are based on an air volume of 24 L and a solvent desorption volume of 2 mL. Air concentrations listed in ppm are referenced to 25°C and 760 mm Hg.)
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 12 ng for phenol and 14 ng for cresol per injection. This is the amount of analyte which will give a peak whose height is 5 times the height of the baseline noise. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 0.97 µg per sample (0.041 mg/m3 or 0.01 ppm) for phenol and 1.1 µg per sample (0.046 mg/m3 or 0.01 ppm) for cresol. This is the amount of analyte spiked on the sampling device which allows recovery of an amount of analyte equivalent to the detection limit of the analytical procedure.(Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 0.97 µg per sample (0.041 mg/m3 or 0.01 ppm) for phenol and 1.1 µg per sample (0.046 mg/m3 or 0.01 ppm) for cresol. This is the smallest amount of analyte which can be quantitated within the requirements of 75% recovery and 95% confidence limits of ±25%. (Section 4.3.)
1.2.4. Sensitivity
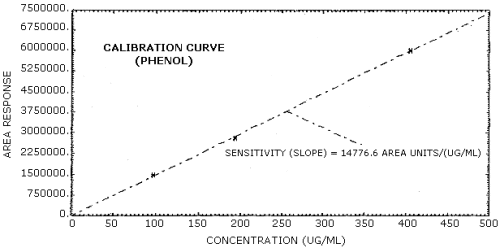
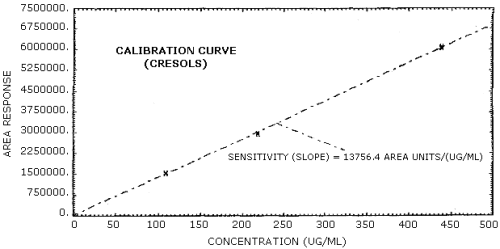
The sensitivity of the analytical procedure over a concentration range representing 0.5 to 2 times the target concentration based on the recommended air volume is 14,777 area units/(µg/mL) for phenol and 13,756 area units/(µg/mL) for cresol. The sensitivity is determined from the slope of the calibration curve. The sensitivity may vary with instruments or instrumental conditions. (Section 4.5.)
1.2.5. Recovery
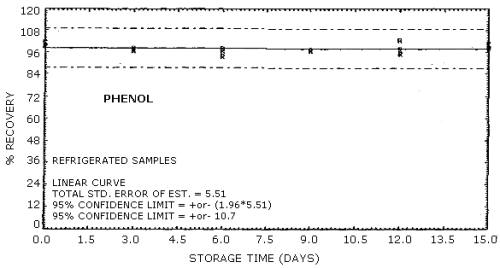
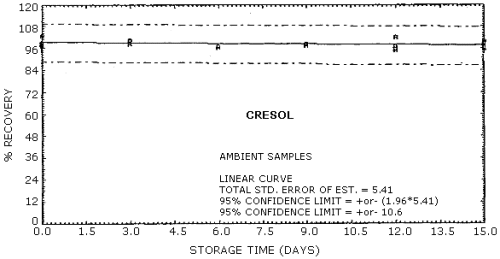
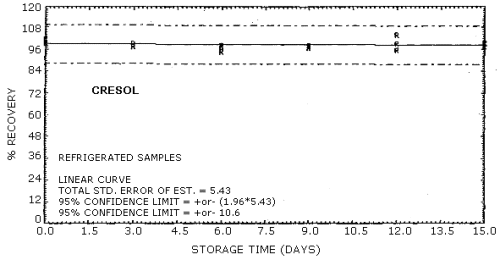
The recovery of the analyte from the collection medium during storage must be 75% or greater. The recovery of phenol and cresol samples stored at ambient conditions for 15 days remained above 93% and 94% respectively. (Section 4.8.)
1.2.6. Precision (analytical procedure)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1 and 2 times the target concentration is 0.0044 for phenol and 0.0061 for cresol. (Section 4.4.)
1.2.7. Precision (overall procedure)
The overall procedure must provide results at the target
concentration that are ±25% or better at the 95% confidence level.
The precision at the 95% confidence level for the
1.3. Advantages
- 1.3.1. The solid sorbent sampling tube for phenol and cresol
provides greater ease of sampling than an aqueous bubbler.
1.3.2. The analysis for phenol and cresols is rapid, sensitive, and precise.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. Use a personal sampling pump which can be calibrated to
within ±5% of the recommended 0.1 L/min flow rate while the sampling
tube is in line.
2.1.2. Use glass sampling tubes of approximately 4 to 5 cm in
length (4-mm i.d. ×
2.2. Reagents
None required
2.3. Technique
- 2.3.1. Label sampling tubes prior to sampling.
2.3.2. Attach the sampling tube to the pump using a section of flexible, plastic tubing. Do not place any tubing ahead of the sampling device. Attach the sampling device in the workers breathing zone in such a manner that it does not impede work performance.
2.3.3. After sampling for the appropriate time, remove the sampling device, and cap and seal the sampling tube with plastic caps.
2.3.4. Include at least one blank for each sampling set. The blank should be handled in the same manner as the samples with the exception that air is not drawn through it.
2.3.5. Any bulk samples submitted for analysis must be shipped in separate containers to avoid contamination of the air samples.
2.3.6. List any potential interferences on the sample data sheet.
2.4. Breakthrough
The volume of air containing 35.3 mg/m3
phenol and 34.8 mg/m3 cresols at 80%
relative humidity which can be sampled at 0.2 L/min before 5% of the
total analytes collected is detected on the backup section of the
sampling tube is estimated to be 173 L for phenol and 216 L for
cresol. These breakthrough volumes are based on two of three
breakthrough studies using
2.5. Desorption efficiency
The desorption efficiency of the analytes from the collection medium must be 75% or greater. The average desorption efficiency over the range of 0.5 to 2 times the target concentration is 99.6% for phenol and 97.9% for cresol. (Section 4.6.).
2.6. Recommended air volume and sampling rate
A 24-L air sample obtained by sampling at 0.1 L/min for 4 h is recommended for phenol and cresol. If necessary, the sensitivity of the analytical method will permit a sampling period as short as 15 min at 0.1 L/min for determination of the analytes at the target concentration.
2.7. Interferences
There are no known interferences to the sampling procedure.
2.8. Safety precautions
- 2.8.1. Attach the sampling equipment to the worker in such a
manner that it will not interfere with work performance or safety.
2.8.2. Follow all safety practices that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A high performance liquid chromatograph equipped with
sample injector, analytical reverse-phase HPLC column, variable
wavelength detector, chart recorder and all necessary hardware
needed for the analysis. A Waters 6000A pump, a Waters WISP 710 auto
sampler, a Perkin-Elmer LC-55 UV-Visible detector and a stainless
steel column (25-cm length × 4.
3.1.2. An electronic integrator or other suitable means of measuring detector response is required. A Hewlett-Packard 3354 data system was used in this study.
3.1.3. Various sizes of volumetric glassware and pipettes are needed for sample and standard preparations.
3.1.4. Three-milliliter (or larger) screw-cap or crimp-type vials
are needed for desorbing the
3.1.5. Small brown glass bottles fitted with inert cap liners are needed to store standard solutions.
3.2. Reagents
- 3.2.1. HPLC grade methanol.
3.2.2. HPLC grade water. Our laboratory uses a commercially available water filtration system for the preparation of HPLC grade water.
3.2.3. Reagent grade phosphoric acid.
3.2.4. Reagent grade standards of phenol and the cresol isomers are required. The standards used in this study and their source are listed below:
- Phenol, Chem. Service, (Lot 0-879), (West Chester, PA.);
3.3. Standard preparation
- 3.3.1. Prepare a stock solution of phenol by weighing
approximately 120 mg of phenol into a 25-mL volumetric and diluting
to volume with methanol. Prepare stock solutions of each of the
cresol isomers by weighing approximately 35 mg of each isomer into
separate 25-mL volumetrics and diluting to volume with methanol.
3.3.2. Prepare 1/50, 1/25, and 2/25 dilutions of phenol and of each of the cresol isomers into the appropriate volumes of methanol to yield standard mixtures of phenol and the cresol isomers which represent 0.5, 1, and 2 times the target concentration. Transfer the standards to dark brown glass bottles fitted with Teflon-lined caps for storage in the refrigerator.
3.4. Sample preparation
Transfer the front glass wool and sorbent section of the sampling tube to a 4-mL vial. Add 2 mL of methanol, immediately cap the vial, and shake it on a mechanical shaker for 15 min. Place the remaining backup section including both glass wool plugs into a separate 4-mL vial and desorb the sample in the same manner as the front sections.
3.5. Analysis
- 3.5.1. Prepare a high performance liquid chromatograph for
sample analysis using the HPLC conditions listed below:
| column: | (25 cm × 4. |
| mobile phase: | 59/41 (v/v) methanol/water, 0.1% H3PO4 (v/v) |
| flow rate: | 1 mL/min |
| UV detector: | 218 nm |
| injection volume: | 25 µL |
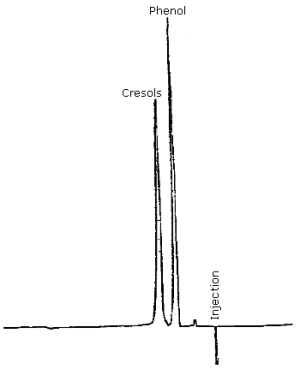
| retention time: | phenol = 5.2 min cresol isomers = 6.9 min |
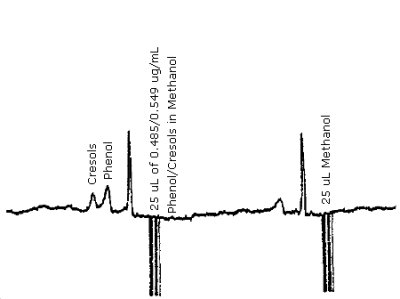
| chromatogram: | Figure 4.9. |
Insure that both the front and back sections of all sampling tubes are analyzed. Verify that all sample response values lie within the range of the responses observed for the standards.
3.5.2. The individual cresol isomers are not resolved by this method. A complete resolution of the three isomers in a tar acid mixture has been accomplished using normal phase HPLC methods although the analysis time is 30 to 40 min (Ref. 5.2.). It is not necessary to resolve the cresol isomers in the analysis since the permissible exposure limit makes no distinction between isomers, and an equal response of the isomers is obtained at 218 nm. It must be recognized that analysis of a cresol sample at wavelengths other than 218 nm can produce erroneous results if the weight ratio of cresol isomers in a sample differs markedly from the ratio in an analytical standard. An equal weight ratio of ortho-, meta-, and para-cresol isomers was used in this study.
3.5.3. Analysis of phenol and cresol by gas chromatography (GC) with flame ionization detection provides a good alternate analytical method. Although somewhat less sensitive than UV detection, the GC analysis does provide a better separation of the cresol isomers (Figure 4.10.).
| GC conditions | |
| column: | (6 ft × 1/8 in.) stainless steel column packed with 0.1% SP1000 on 80/100 Carbopack C |
| injector: | 225°C |
| detector temp.: | 225°C |
| detector gases: | 250 mL/min, air; 20 mL/min, H2 |
| oven: | 210°C |
| N2 carrier gas: | 20 mL/min |
3.6. Interferences
Any compound which has the same retention time as phenol or cresol is a potential interference. Comparisons of the peak height ratios of analyte response obtained at two wavelengths for both samples and standards is a valuable confirmatory technique in HPLC. This technique can be applied to the analysis of phenol but not to the unresolved cresol isomers since different isomeric mixtures of standard and sample will give different wavelength ratios. Analysis by GC offers an excellent means of sample confirmation for both phenol and the cresols. A comparison of the results of an analysis of Beechwood creosote by both methods is given below:
Lot #11946 Beechwood Creosote
|
| ||
| HPLC analysis | GC analysis | |
|
| ||
| % phenol % cresol |
6.55% 19.3% |
6.91% 19.7% |
|
| ||
3.7. Calculations
- 3.7.1. Prepare a standard calibration curve of area response
versus concentration for both of the analytes. Calculate the analyte
concentration in the samples using a
3.7.2. Include in the calculations the concentration of the analytes found on the backup section of a sampling tube. Express results in mg/m3 using the following equation:
mg/m3 = (µg/mL)(2 mL desorption)/(air volume in liters)
To convert to ppm at 760 mm and 25°C:
ppm
= (mg/m3)(24.46)/(MW of analyte)
24.46 is the molar volume of an ideal gas at 760 mm Hg and 25°C
3.8. Safety precautions
- 3.8.1. Minimize exposure to phenol and cresol vapors by
performing standard preparations in a well ventilated hood.
3.8.2. Avoid all skin contact with phenol and cresol.
3.8.3. Restrict the use of solvents to well ventilated hoods.
3.8.4. Wear safety glasses in laboratory areas at all times.
4. Backup Data
- 4.1. Detection limit for analytical procedure
The detection limit for the analytical procedure is 12 ng for phenol and 14 ng for cresol. This is based on a 25 µL injection of a 0.485 ng/µL phenol and 0.549 ng/µL cresol standard mixture. A chromatogram of the detection limits of the analytical procedure for phenol and cresol are given in Figure 4.1.
4.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 0.97 µg per sample (0.041 mg/m3 or 0.01 ppm) for phenol and 1.1 µg per sample (0.046 mg/m3 or 0.01 ppm) for cresol. This is based on the presence of 0.485 µg/mL of phenol and 0.549 µg/mL of cresol in 2 mL of desorbing solution.
4.3. Reliable quantitation limit
The reliable quantitation limit is the same as the detection limit
of the overall procedure since the recovery at this concentration is
at least 75% and the precision is ±25% or better at the 95% confidence
level. The front section of four
Reliable Quantitation Limit Data
|
| ||
| analyte µg/sample |
phenol 0.971 |
cresol 1.10 |
|
| ||
| µg recovered SD(%) 1.96SD(%) |
0.750 0.988 0.868 0.868 0.868(89) 0.097(11.2) 0.190(22) |
0.824 0.824 0.952 1.06 0.915(83) 0.115(12.6) 0.225(25) |
|
| ||
4.4. Precision
The pooled coefficients of variation over a range of 0.5 to 2 times the target concentration for both phenol and cresol were obtained from multiple 25-µL injections of three standard mixtures in methanol containing 97.08/109.8, 194.2/219.6, and 405.1/439.3 µg/mL of phenol and cresol respectively. The results are listed in Table 4.4.1. and 4.4.2.
Analytical Precision Data for Phenol
|
| |||
| (µg/mL) | 97.08 | 194.2 | 405.1 |
|
| |||
| area response ×
10-6 SD CV |
1.487 1.488 1.466 1.482 1.486 1.490 1.483 0.0088 0.0059 |
2.822 2.809 2.801 2.790 2.798 2.810 2.805 0.0111 0.0040 |
6.033 6.003 6.004 6.022 5.985 6.012 6.010 0.0166 0.0028 |
|
| |||
Analytical Precision Data for Cresol
|
| |||
| (µg/mL) | 109.8 | 219.6 | 439.3 |
|
| |||
| area response ×
10-6 SD CV |
1.558 1.563 1.537 1.545 1.569 1.568 1.557 0.013 0.0084 |
3.013 2.990 3.003 2.979 2.981 3.008 2.996 0.014 0.0048 |
6.113 6.043 6.081 6.098 6.059 6.070 6.078 0.025 0.0041 |
|
| |||
4.5. Sensitivity
The slope of the calibration curve over the range of 0.5 to 2 times the target concentration for the analytes represents the sensitivity of the method. The sensitivities determined in this manner are 14,777 and 13,756 area units/(µg/mL) respectively for phenol and cresol. (Figures 4.5.1. and 4.5.2.)
4.6. Desorption efficiency
The desorption efficiency from spiked samples over the range of 0.5
to 2 times the target concentration is 99.6% for phenol and 97.9% for
cresol. A total of 18
Desorption Efficiency for Phenol
|
| |||
| × target conc. phenol, µg |
0.5× 242 |
1× 465 |
2× 893 |
|
| |||
| desorption efficiency, % |
101 101 103 98.0 100 101 101 |
99.0 100 99.0 100 99.0 99.1 99.3 |
96.8 100 101 99.6 97.3 96.9 98.6 |
|
| |||
Desorption Efficiency for Cresol
|
| |||
| × target conc. phenol, µg |
0.5× 242 |
1× 465 |
2× 893 |
|
| |||
| desorption efficiency, % |
95.8 95.8 96.5 96.5 95.1 96.0 96.1 |
97.1 98.1 98.1 97.1 96.1 98.1 97.4 |
98.4 102 103 101 98.7 98.7 100.3 |
|
| |||
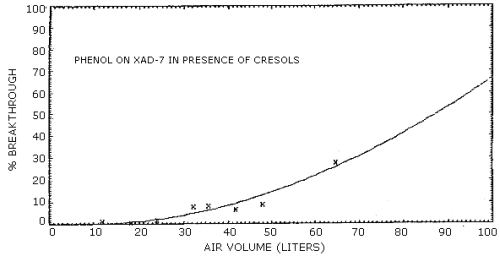
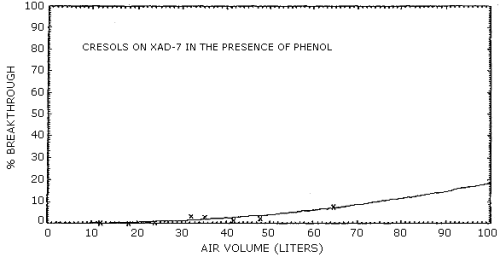
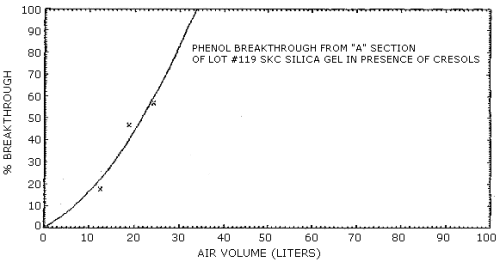
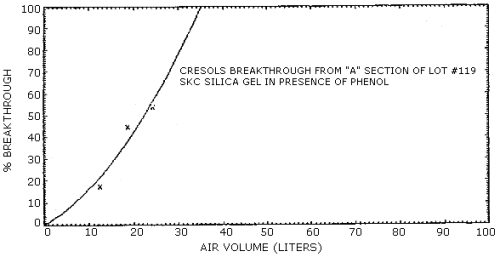
4.7. Breakthrough
Commercially available Tenax, Silica gel,
The retention efficiency, which is the ability to retain the analytes when humid air was drawn through a sampling tube, was determined for all of the sorbent materials except Tenax. These retention efficiencies were measured by drawing humid air (80% RH) at 0.1 L/min, for a minimum of 3 h, through sorbent tubes which were spiked with an amount of phenol and cresol equivalent to twice the target concentration. Silica gel was the only sorbent material which failed to adequately retain the analytes on the front adsorbent section of the sampling tube.
Further evaluations of a collection method for phenol and cresol were performed using a vapor generation system. A 1.00% aqueous solution of phenol and a 0.985% aqueous solution of an equal-weight mixture of the cresol isomers were metered into a 2 L/min airstream with separate 10-mL syringes at flow rates of 7.56 µL/min and 7.72 µL/min respectively. A constant 120°C temperature was maintained at the inlet to the vapor generation system by wrapping the inlet with heating tape to ensure rapid volatilization of the analytes. Based on the analysis of sampling tubes used to monitor the generated atmosphere, approximately 92% of the expected concentration of 37.8.0 mg/m3 phenol and 38.0 mg/m3 cresol was obtained with the system.
Attempts to monitor analyte breakthrough using either a total
hydrocarbon analyzer, or a gas chromatograph equipped with a gas
sampling valve mounted downstream from the sampling tube were
unsuccessful. It is suspected that adsorption of the analytes onto the
glass surfaces of the vapor generation system resulted in the long lag
time observed between actual breakthrough and the time required for
detection of breakthrough. Reliable measures of breakthrough were
determined by analyzing both the front and back sections of solid
sorbent sampling tubes placed in the vapor stream for various lengths
of time. A maximum of six sampling tubes could be placed on the
sampling manifold at one time. Critical flow orifices attached between
the sampling tubes and the vacuum system were used to accurately
sample the test atmosphere at 0.2 L/min. With only one exception, all
breakthrough studies were performed at approximately 80% relative
humidity. Breakthrough was measured by removing the sampling tubes
from the vapor stream at various time intervals and then analyzing
front and back sorbent sections including the glass wool plugs. The
results for the various solid sorbents tested were compared by
plotting the air volume sampled versus the percent of the total
analyte found on the backup section. A least squares parabolic curve
forced through zero was arbitrarily used to fit the data points. The
air volumes necessary to give 5, 10, 15, and 20 percent breakthrough
were determined from the equations for the curves. Representative
breakthrough curves for
Due to the high analyte capacities observed for most of the sorbents tested, less than normal amounts of sorbent material were generally used in the breakthrough studies. Unless otherwise indicated, all of the breakthrough studies were performed with an accurately weighed 25-mg front portion of adsorbent and an approximate 50-mg back portion. Small silanized glass wool plugs were used to separate and retain the sections.
The results of breakthrough studies for all solid sorbents tested
are presented in Tables 4.7.1. - 4.7.4. Breakthrough tests on
An initial screening of SKC, Inc. Tenax (2,6-diphenyl-p-phenylene oxide polymer) tubes indicated that this material was not effective in trapping the analytes and further evaluations of breakthrough were not conducted. The sampling of 36 L of the phenol and cresol atmosphere at 0.2 L/min and at 80% relative humidity resulted in the retention (on the entire Tenax sampling tube) of only 22% of the total phenol and 56% of the total cresol present in the atmosphere.
The capacities of the various sorbents on a percent weight basis are reported in Tables 4.7.5. and 4.7.6. These values were determined from the breakthrough studies by dividing the amount of analyte on the front section of the sampling tube at saturation by the weight of the solid sorbent used. With the possible exception of silica gel, all of the sorbents demonstrated a higher capacity for cresol than for phenol. These values are consistent with the differences in breakthrough air volumes observed for cresol and phenol. The low capacities measured for silica gel and Tenax are also reflected in low breakthrough air volumes for these adsorbents.
Examination of the breakthrough volume and the capacity data
indicate that
The breakthrough volumes and the capacities determined for the PoraPak resins indicate that these sorbent materials are also quite effective in collecting the analytes. The SKC PoraPak sampling tubes were not selected for use because they presented some potential sampling and analytical problems. The fine mesh size of the PoraPak resins used in the SKC sampling tubes resulted in a large pressure drop of 5 inches of water across the sampling tube at a 0.1 L/min flow rate. This may affect sample pump performance during prolonged sampling periods. Some problems were also experienced with the analysis of the PoraPak sampling tubes. Difficulty in transferring the resins for methanol extraction was experienced, and extraneous UV-absorbing peaks extracted from the resins were observed upon analysis. All of the problems associated with the PoraPak resins might easily be overcome if properly sized and properly solvent-extracted resins are used in the sampling tubes.
Parameters for Tests listed in Table 4.7.2.
|
| |||
analyte |
relative humidity (%) |
amount of adsorbent (mg) | |
|
|
|||
| test 1 test 2 test 3 test 4 test 5 test 6 test 7 |
phenol/cresol phenol/cresol phenol/cresol phenol/cresol phenol phenol cresol |
80 80 80 50 80 80 80 |
25 30* 25 25 25 25 25 |
|
| |||
| * Air volumes of test 2 corrected by weight difference factor. (25 mg/30 mg) × air vol. | |||
Brekathrough (BT) Air Volumes (L) on
|
| |||||||
%BT |
1 phen/cres |
2 phen/cres |
3 phen/cres |
4 phen/cres |
5 phen |
6 phen |
7 cres |
|
| |||||||
| 5 10 15 20 |
32.1/54.1 42.9/* 50.9/* 57.8/* |
38.2/54.1 46.0/67.9 52.0/78.8 57.7/* |
15.0/28.7 24.0/40.9 31.3/50.2 37.5/58.0 |
53.7/58.9 45.1/77.5 52.5/* 58.8/* |
29.6 39.0 46.3 52.4 |
27.2 37.0 45.1 52.2 |
44.2 56.8 66.7 75.0 |
|
| |||||||
| * No breakthrough data obtained at
this level. test atmosphere conc.: phenol - 37.8 mg/m3, cresol - 38.0 mg/m3 sampling rate: 0.2 L/min | |||||||
Phenol Breakthrough (BT) Air Volumes (L) for Solid Sorbents
|
| |||||||
| %BT |
PoraPak R |
PoraPak S |
PoraPak1 T (30 mg) |
PoraPak T |
Silica
Gel1 (140 mg) | ||
|
| |||||||
| 5 10 15 20 |
28.3 36.6 43.1 48.6 |
23.8 32.8 39.7 45.6 |
34.6 41.7 47.5 52.4 |
41.8 49.5 55.8 61.2 |
5.2 11.0 17.4 24.9 |
15 25.5 34.1 41.6 |
0.68 1.20 1.64 2.04 |
|
| |||||||
| 1
Air volumes of test 2 corrected by weight difference
factor. (25 mg/mg used in test) × air vol. | |||||||
Cresol Breakthrough (BT) Air Volumes (L) for other Sorbents
|
| ||||||
| %BT | PoraPak R | PoraPak S | PoraPak T1 | PoraPak T | ||
|
| ||||||
| 5 10 15 20 |
52.7 67.7 79.3 * |
49.7 62.6 72.8 * |
50.5 62.1 71.4 * |
65.5 * * * |
37.0 * * * |
31.4 * * * |
|
| ||||||
| 1
Thirty milligrams used in test. Air volumes corrected by weight
difference factor. (25 mg/30 mg) × air vol. * No breakthrough data obtained at this concentration. | ||||||
Capacity1 of Phenol and Cresol on
|
| ||
| test | phenol | cresols |
|
| ||
| 1 2 (30 mg) 3 4 (50% RH) 5 (phenol only) 6 (phenol only) 7 (cresol only) |
5.7 5.2 4.8 5.9 5.6 5.8 - |
7.7 7.3 6.8 7.9 - - 7.8 |
|
| ||
| 1 percent by weight | ||
Capacity1 of Other Sorbents
|
| ||
| test | phenol | cresols |
|
| ||
| PoraPak T PoraPak T (30 mg) PoraPak R PoraPak S silica gel Tenax |
5.7 5.0 4.8 5.0 4.2 4.4 0.2 0.5 |
8.0 7.3 8.1 7.9 6.4 5.4 0.31 1.5 |
|
| ||
| 1 percent by weight | ||
4.8. Storage data
No stability problems were observed upon storage of phenol and
cresol on
Phenol Storage Tests
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 3 6 9 12 15 |
99.8 97.6 98.2 97.3 103 98.7 |
102 96.9 96.9 97.2 95.2 101 |
99.6 98.3 93.8 97.8 97.7 98.0 |
97.9 99.8 99.4 99.2 97.0 96.9 |
103 99.2 96.7 97.9 102 99.5 |
97.3 98.2 96.0 95.9 93.8 99.5 | |
|
| |||||||
Cresol Storage Tests
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 3 6 9 12 15 |
99.8 97.5 97.8 97.7 104 97.2 |
102 98.2 98.6 97.0 95.9 99.7 |
99.3 99.7 95.1 98.4 99.3 99.5 |
97.4 98.4 96.4 98.0 96.5 96.1 |
103 99.5 96.5 97.8 102 98.3 |
98.4 100 96.4 97.7 94.8 99.6 | |
|
| |||||||
5. References
- 5.1. Donald McNeil in "Kirk-Othmer Encyclopedia of Chemical
Technology", Vol. 6, pp. 434 - 444, 2nd Edition, John Wiley and Sons,
N.Y. 1965.
5.2. Husain, S.; Kunzelmann, P.; Schildknecht, H.; J. Chromatogr. (1977), 137, 53 - 60.
5.3. "NIOSH Manual of Analytical Methods", Vol. 3, 2nd Edition, April 1977, USDHEW, PHS, CDC, NIOSH, DHEW (NIOSH) Publication No. 77 - 157C.
5.4. "Documentation of the NIOSH Validation Tests", by Taylor, D.G.; Kupel, R.E.; and Bryant, J.M.; USDHEW, PHS, CDC, NIOSH, April 1977, DHEW (NIOSH) Publication No. 77 - 185.
5.5. Kuwata, K.; Uebori, M.; Yamazaki, Y.; Anal. Chem. (1980), 52, 857860.
5.6. "Criteria for a Recommended Standard....Occupational Exposure to Phenol", USDHEW, PHS, CDC, NIOSH, July 1976, HEW Publ. No. (NIOSH) 76 - 196.
5.7. "Criteria for a Recommended Standard....Occupational Exposure to Cresol", USDHEW, PHS, CDC, NIOSH, Feb. 1978, DHEW (NIOSH) Publication No. 78 - 133.
5.8. Dave, S.B.; J. Chromatog. Sci., (1969), 7, 389 - 399.