MDI AND TDI BY THERMAL ENERGY ANALYSIS
| Method no.: | 33 |
| Recommended minimum sample concentration for confirmation: |
1 µg/sample MDI or TDI |
| Procedure: | Air samples are collected and analyzed as recommended
in OSHA Organic Division Method No. 18 - Diisocyanates: 2,4-TDI and
MDI (Ref. 4.1.). Following routine analysis, samples which exceed
the OSHA PEL are submitted in a graduated evaporative concentrator
for confirmation. The sample is evaporated to dryness and rediluted
with chloroform. Excess nitro reagent is removed by extraction and
the sample is subjected to |
| Minimum sample concentration required for detection: |
0.13 µg/sample for TDI |
| 0.15 µg/sample for MDI | |
| Status of method: | A confirmatory procedure which has been developed and reviewed by the Organic Methods Evaluation Branch. |
| Date: November 1981 | Chemist: Warren Hendricks |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Introduction
The OSHA air sampling procedure for toluene-2,4-diisocyanate (TDI)
and methylene bisphenyl isocyanate (MDI) recommends the use of a
bubbler containing 15 mL of 0.0002M
The OSHA procedure for the routine analysis of the nitro reagent
derivatives of MDI and TDI (MDIU and TDIU) recommends using
Mass spectrometric (MS) confirmation of air samples which exceed the OSHA PEL is often difficult because the MS method has a relatively high detection limit and the mass spectra are usually complicated. Because the MS procedure uses the direct insertion probe (DIP) method to introduce the sample, the analyst must isolate and concentrate the analytes by repetitive HPLC separation and peak collection. This process is time consuming and usually inconvenient.
The highly selective Thermal Energy Analyzer (TEA) has been in use
to determine
When the analytes were subjected to separation by gas
chromatography (GC) nitro reagent, MDIU and TDIU all gave a single
sharp peak at the same retention time on a 3-ft glass
The effects of increasing the GC pyrolyzer temperature on detector
response were studied. It was found that maximum detector response for
the analytes occurred at 875
The maximum temperature that the HPLC pyrolyzer can attain is
800
Because, at this time, it does not seem possible to separate and
then simultaneously confirm the analyte at sufficiently low levels, a
compromise method is recommended. More than 99% of the excess nitro
reagent in air samples can be removed by a simple acid extraction.
Following nitro reagent removal, the sample is separated into its
components by
It is unlikely that an interference will have the same retention
time on both reversed and
Data have been collected on MDI and TDI samples subjected to
This alternative method is not intended for routine analytical use.
It was developed to confirm high results obtained by the routine
1.2. Detection limit of the analytical procedure (GC/TEA)
The detection limit of the GC/TEA analytical procedure is 81 pg for TDI and 92 pg for MDI per GC/TEA injection. This is the amount of analyte which will give a peak whose height is about five times the height of the baseline noise (Section 3.1.).
1.3. Minimum sample concentration required for detection
The minimum sample concentration required for detection is 0.13 µg per TDI sample and 0.15 µg per MDI sample. This is equivalent to 7 µg/m3 for TDI and 8 µg/m3 for MDI based on the recommended air volume of 20 L.
1.4. Advantages
- 1.4.1. This method has a lower detection limit than the MS/DIP
procedure.
1.4.2. This procedure is less tedious than the MS/DIP method because it eliminates the need for multiple HPLC runs to isolate and concentrate the analyte.
1.4.3. The cost of the recommended instrumentation is less for this method than for the MS/DIP procedure.
1.4.4. It is possible to quantitate results obtained by use of this method.
1.5. Disadvantages
- 1.5.1. The analytes can not be simultaneously separated and
confirmed by use of this method.
1.5.2. Unlike the MS/DIP procedure, the molecular structure of the compound in question is not obtained through use of this method.
2. Analytical Method
- 2.1. Apparatus
- 2.1.1. HPLC apparatus equipped with UV detector, sample injector
and chart recorder. The UV detector used in this work was a Waters
Associates Model 440 Absorbance Detector. The detector was equipped
with a 50-cm length of 0.23-mm i.d. stainless steel tubing attached
to the outlet of the sample cell for peak collection.
2.1.2. HPLC analytical column capable of separating MDIU and
TDIU. The column used in this work was a
2.1.3. Electronic integrator or other suitable means to determine peak areas.
2.1.4. Graduated evaporative concentrators, 10 mL, Kontes or equivalent.
2.1.5. Temperature controlled water bath equipped with nitrogen stream evaporative needles.
2.1.6. Vortex mixer, Scientific Products Deluxe Mixer S8220 or equivalent.
2.1.7. Laboratory centrifuge, IEC HN-SII Centrifuge, or equivalent.
2.1.8. Vials, 2-mL with Teflon-lined caps.
2.1.9. Gas chromatograph.
2.1.10. Thermal Energy Analyzer equipped with an Explosives Analysis Package, Thermo Electron Corp., Waltham, Mass.
2.1.11. GC column capable of resolving the analyte decomposition
product from potential interferences. The column used in this work
was 3 ft ×
2.1.12. Dewar flasks, for liquid nitrogen.
2.1.13. Pipets, disposable Pasteur type.
2.1.14. Assorted miscellaneous laboratory equipment.
2.1.15. Stopwatch.
2.2. Reagents
- 2.2.1. Analytical standards, see Section 3.3. of OSHA Organic
Division, Method No. 18, Diisocyanates: 2,4-TDI and MDI (Ref. 4.1.).
2.2.2. Methanol, isopropanol, isooctane, toluene, acetonitrile,
chloroform, and
2.2.3. Phosphoric acid, 1% in deionized water by volume, reagent grade.
2.2.4. Liquid nitrogen.
2.2.5. Helium and nitrogen, GC grade.
2.2.6. Oxygen, medical grade.
2.3. Standard preparation
- 2.3.1. Prepare MDIU and TDIU standards, diluted with
acetonitrile, as described in Section 3.3. of OSHA Organic Division,
Method No. 18 Diisocyanates: 2,4-TDI and MDI (Ref. 4.1.).
2.3.2. Place 1.00 mL of each standard from the working range into
a 10-mL concentrator tube. Evaporate the standard to dryness using a
heated water bath (55
2.3.3. Allow the concentrator tube to return to room temperature and then add 1.00 mL of chloroform.
2.3.4. Add 5 mL of 1% v/v phosphoric acid to the concentrator tube and then mix the contents of the tube using a vortex mixer for 30 seconds. The phosphoric acid serves to extract nitro reagent from the organic to the aqueous phase.
2.3.5. Separate the aqueous and organic phases by centrifuging the concentrator tube.
2.3.6. Remove and discard the aqueous (upper) phase with a disposable pipet. Using a clean pipet, transfer the organic (lower) phase to a small vial and then tightly seal the vial with a Teflon-lined cap. Be careful not to transfer aqueous with the organic phase. The standard is now ready for HPLC/UV analysis and component isolation.
2.4. Sample preparation
About 0.5 mL of each sample to be confirmed should be submitted in a graduated evaporative concentrator. The sample should contain at least 1 µg of analyte. The person requesting the confirmation should provide the suspected concentration and identity of the analyte in question. The sample should be stored in a freezer until analysis.
- 2.4.1. Record the volume of the sample in the graduated
concentrator to two decimal places. Evaporate the sample to dryness
using a heated (55
2.4.2. Allow the concentrator tube to return to room temperature and then add 1.00 mL of chloroform. If the sample to be confirmed contains low levels of the analyte, 0.50 mL of chloroform may be substituted to give a more concentrated solution.
2.4.3. Add 5 mL of 1% v/v phosphoric acid to the concentrator tube and then mix the contents of the tube using a vortex mixer for 30 s. The phosphoric acid serves to extract nitro reagent from the organic to the aqueous phase.
2.4.4. Separate the aqueous and organic phases by centrifuging the concentrator tube.
2.4.5. Remove and discard the aqueous (upper) phase with a disposable pipet. Using a clean pipet, transfer the organic (lower) phase to a small vial and then tightly seal the vial with a Teflon-lined cap. Be careful not to transfer aqueous with the organic phase. The sample is now ready for HPLC/UV analysis and component isolation.
2.5. HPLC/UV analysis
- 2.5.1.
| column: | Dupont Zorbax CN (25 cm × 4.6 mm) or equivalent |
| mobile phase: | 75/15/10 (v/v/v) isooctane/isopropanol/methanol |
| flow rate: | 1 mL/min |
| UV detector: | 254 nm (fixed wavelength) |
| injection volume: | 25 µL |
| chromatogram: | Figure 3.2. |
2.5.2. HPLC separation and peak collection
- 2.5.2.1. Determine the retention time for each analyte using
standards of similar concentration as those suspected in the
samples.
2.5.2.2. Isolate each analyte by collection of the HPLC column effluent at the appropriate time using a 10-mL graduated concentrator tube. The use of excessive tubing and/or valves to collect the analyte is not recommended. The 50-cm length of tubing described in Section 2.1.1. has a dead volume of 21 µL and the transfer time from the sample cell to the collection point is 1 s. Therefore, the transfer time from the sample cell to the collection point is insignificant when the recommended apparatus is used.
2.5.2.3. Evaporate the collected analyte to dryness using a
heated (55
2.5.2.4. Allow the concentrator tubes to return to room temperature and then add 0.20 mL toluene to each tube. Mix the contents of each tube using a vortex mixer.
2.5.2.5. Reinject each collected standard and sample to insure that proper peak collection technique has been used. When using Zorbax CN analytical column, toluene will not present a chromatographic interference.
2.6. GC/TEA analysis
- 2.6.1. GC conditions
| column: | 3 ft × 1/4-in. o.d. (2-mm i.d.) glass, on-column injection, 10% SP-1000 on 80/100 Supelcoport. |
| injector temperature: | 250 |
| column temperature: | 240 |
| GC/TEA interface temperature: | 250 |
| helium (carrier gas) flow rate: | 30 mL/min |
| injection volume: | 5 µL |
2.6.2. TEA conditions
| GC pyrolyzer temp.: | 875 |
| oxygen flow rate: | 0.5 mL/min |
| pressure: | 0.5 mm Hg |
| cold trap temp.: | -130 |
| chromatogram: | Figure 3.3. |
2.7. Analysis notes
Results of this method are quantitative. Confirmation of suspected
MDIU and TDIU in air samples depends on the comparison of results
obtained by the
- 2.7.1. Measure UV and TEA detector response with an electronic
integrator or other suitable means.
2.7.2. Compare samples to standards of similar concentration. This is easy to do because the suspected concentration of samples is known prior to confirmation.
2.7.3. Use an external standard procedure to prepare a calibration curve using at least three standard solutions of different concentrations. Prepare the calibration curve daily. Calibrate the integrator to report results in µg/mL.
2.8. Interferences
- 2.8.1. Nitro reagent is an interference in the GC/TEA analysis
of TDIU and MDIU. Excess nitro reagent is removed by phosphoric acid
extraction prior to HPLC/UV analysis. The potential interference of
nitro reagent is further reduced by HPLC separation of the analytes
prior to peak collection. The analysis of blank samples will confirm
the absence of nitro reagent.
2.8.2. Any compound having the same retention time as the
analytes and giving a TEA response is a potential interference.
Generally, HPLC or GC parameters can be changed to circumvent an
interference. An interference can often manifest itself by causing a
difference in expected results. If the
2.9. Calculations
The following section applies to both HPLC/UV and GC/TEA results.
- 2.9.1. Use the integrator value, in µg/mL, for reference only.
More reliable results are obtained by use of a calibration curve.
The detector response, for each standard, compared to its equivalent
concentration in µg/mL and the best straight line through the data
points is determined by linear regression.
2.9.2. Determine the concentration, in µg/mL, for a particular sample by comparing its detector response to the calibration curve.
2.9.3. HPLC/UV
Corrected µg/mL diisocyanate =
| µg/mL from Section 2.9.2. × | volume from Section 2.4.2.
volume from section 2.4.1. |
2.9.4. GC/TEA
Corrected µg/mL diisocyanate =
| µg/mL from Section 2.9.2. × | volume from Section 2.4.2.
volume from section 2.4.1. |
2.9.5. Discussion
If the
2.10. Safety precautions
- 2.10.1. Sample and standard preparations should be done in a
fume hood. Avoid exposure to diisocyanates.
2.10.2. Avoid skin contact with liquid nitrogen and the solvents.
2.10.3. Avoid exposure to solvent vapors.
2.10.4. Wear safety glasses in all laboratory areas.
2.10.5. Check to be sure that the TEA exhaust is connected to a fume hood.
3. Backup Data
The chromatograms in this section were generated by the analysis of MDIU and TDIU, however, all calculated results and amounts were presented as free MDI and TDI.
- 3.1. Detection limit of the analytical procedure (GC/TEA)
The GC/TEA chromatogram shown in Figure 3.1. represents the
detection limit for TDIU and MDIU. Twenty-five microliters of an acid
extracted standard containing 0.13 µg/mL TDI in chloroform was
subjected to
3.25 ng/0.20 mL = 16.25 ng/mL TDI
5 µL × 16.25 ng/µL TDI = 81 µg TDI
Therefore, the GC/TEA detection limit for TDI is 81 µg per injection.
Because the TEA response is molar, the detection limit for MDIU may be calculated.
| 638 (MW for MDIU)
562 (MW for TDIU) |
× 81 pg TDI = 92 pg MDI |
The detection limit for MDIU is 92 µg per injection.
The detection limit is that amount of analyte which will give a peak whose height is about 5 times the height of the baseline noise.
3.2. Minimum sample concentration required for detection
The following sample concentrations will provide the necessary quantities for GC/TEA detection and the concentrations are more than adequate for HPLC/UV detection.
The minimum sample concentration required for detection is 0.13 µg/sample for TDI and 0.15 µg/sample for MDI. This is equivalent to 7 µg/m3 for TDI and 8 µg/m3 for MDI based on the recommended air volume.
The volumes recommended in Section 3.1. were used to determine the minimum concentration required for detection.
3.3. GC/TEA chromatogram
Twenty-five microliters of an acid extracted standard containing
2.5 µg/mL TDI in chloroform was subjected to
3.4. The data in Table 3.4. were generated by the GC/TEA analysis of the same sample using different GC pyrolyzer temperatures. The TEA response at 750°C was assigned a value of 1.0 and the response at other temperatures was calculated relative it.
The Effects of GC Pyrolyzer
Temperature on TEA Detector Response
|
| |
| pyrolyzer | TEA |
| temperature, |
response |
|
| |
| 600 | 0.0 |
| 700 | 0.23 |
| 750 | 1.0 |
| 800 | 3.1 |
| 850 | 9.4 |
| 875 | 14 |
| 900 | 12 |
|
| |
3.5. The data in Table 3.5 were obtained from the
Comparison of Diisocyanate Results (µg/mL)
|
| ||||
| sample | analyte | GC/TEA | ||
|
| ||||
| QC | MDIU | 4.0 | 4.4 | 6.91 |
| QC | MDIU | 6.2 | 6.4 | 7.11 |
| QC | MDIU | 8.1 | 8.8 | 10.61 |
| air | TDIU | 5.2 | 3.3 | 3.81 |
| air | TDIU | 3.8 | 2.6 | 2.91 |
| air | TDIU | 0.77 | 0.4 | 0.6 |
| standard | MDIU | 13.5 | 13.3 | 12.6 |
| standard | MDIU | 10.8 | 11.4 | 10.9 |
| standard | MDIU | 8.1 | 9.4 | 8.0 |
| standard | MDIU | 5.4 | 4.9 | 5.0 |
| standard | MDIU | 2.7 | 2.5 | 3.2 |
| standard | TDIU | 0.26 | 0.28 | 0.49 |
| standard | TDIU | 0.79 | 0.95 | 0.67 |
| standard | TDIU | 1.3 | 1.1 | 1.1 |
| standard | TDIU | 2.5 | 2.5 | 2.6 |
| standard | TDIU | 6.6 | 6.6 | 6.6 |
| QC | MDIU | 5.8 | 6.7 | 6.8 |
| QC | MDIU | 3.8 | 3.5 | 4.5 |
| air | MDIU | 16 | 15 | 15 |
| air | MDIU | 26 | 27 | 26 |
| air | MDIU | 3.0 | 3.0 | 3.4 |
| air | TDIU | 4.0 | 6.8 | 5.6 |
|
| ||||
| 1The solvent used for the final dilution was changed from chloroform to toluene because of the volatility of chloroform. | ||||
The reverse-phase result was divided by the
| average | reversed-phase HPLC/UV
normal-phase HPLC/UV |
= 1.06 |
| average | reversed-phase HPLC/UV
GC/TEA |
= 0.972 |
| average | normal-phase HPLC/UV
GC/TEA |
= 0.941 |
When the above calculations were performed on individual samples
which contained more than 1 µg of analyte and were diluted with
toluene, 40 of 42 individual results were within the range of 0.75 to
1.25 (±25%). These data indicate that results from
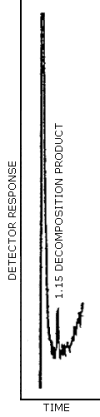
Figure 3.1. GC/TEA detection limit for the decomposition product of
the nitro reagent derivatives of MDI and TDI.
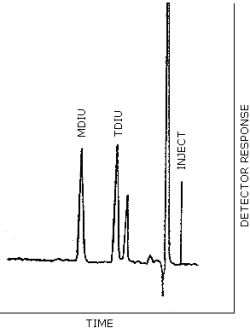
Figure 3.2.

Figure 3.3. GC/TEA chromatogram for the decomposition product of the nitro reagent derivatives of MDI and TDI.
4. References
- 4.1. Cummins, K. Diisocyanates - 2,4-TDI and MDI (Method 18,
Organic Methods Evaluation Branch, OSHA Analytical Laboratory, Salt
Lake City, Utah). Unpublished
4.2. Hendricks, W. Volatile Nitrosamine Mixture I (Method 27,
Organic Methods Evaluations Branch, OSHA Analytical Laboratory, Salt
Lake City, Utah). Unpublished