ACRYLONITRILE |
| Method no.: | 37 |
| Control no.: | T-37-FV-02-0110-M |
| Matrix: | Air |
| Target concentration: | 2 ppm (4.5 mg/m3) (OSHA PEL) |
| Procedure: | Samples are collected on charcoal, desorbed with acetone, and analyzed by gas chromatography using a nitrogen/phosphorus detector. |
| Recommended air volume and sampling rate: |
20 L at 0.2 L/min |
| Detection limit of the overall procedure: |
0.01 ppm (0.026 mg/m3) |
| Reliable quantitation limit: | 0.3 ppm (0.66 mg/m3) |
| Standard error of estimate at the target concentration: (Figure 4.7.2.) |
6.4% |
| Status of method: | A sampling and analytical method which has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch |
| Date: May 1982 | Chemist: Michael Shulsky |
Organic Methods Evaluation Branch
OSHA Analytical
Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1 Background
- 1.1.1 History
A collection and analytical method was needed for acrylonitrile which would be convenient, sensitive, and accurate. This evaluated method, which is similar to a previously published method (Ref. 5.4) consists of collecting acrylonitrile vapor on charcoal, desorbing with acetone containing an internal standard and analyzing by gas chromatography (GC) equipped with a nitrogen/phosphorus detector (NPD).
Two other methods also use charcoal for collection. One recommended by NIOSH (Ref. 5.2) uses methanol for desorption and GC with a flame ionization detector (FID) for analysis. However, methanol does not provide adequate desorption efficiencies at low loadings of acrylonitrile nor is the FID as sensitive or selective for acrylonitrile as the NPD. The second method which uses GC/NPD for analysis (Ref. 5.3), also uses methanol to desorb the charcoal but with sonication to increase the desorption efficiency. Acetone desorption requires no sonication.
Another sampling and analytical (GC) procedure reported in the literature (Ref. 5.1) recommends collection on Porapak N adsorbent and thermal desorption. Thermal desorption is inconvenient when the identity of an analyte must be confirmed by an alternate analytical technique.
1.1.2 Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
Absorption of acrylonitrile may occur through unbroken skin and from the respiratory and gastrointestinal tract. (Ref. 5.5) Acrylonitrile exhibits the toxic characteristics of the cyanide ion, and several researchers have attributed its toxicity to the cyanide ion. But other researchers have concluded that acrylonitrile toxicity is not due only to cyanide because of the ineffectiveness of anti-cyanide drugs. (Ref. 5.5.)
Exposures at 636 ppm in air for 4 h were fatal to rats. Dogs exposed to 50, 75, and 100 ppm levels for 7 h showed histologic changes. (Ref. 5.5.)
An interim report from an oral toxicity study conducted by Dow Chemical Company stated that rats of both sexes which ingested 35, 100 or 300 ppm in their drinking water developed tumors at all acrylonitrile levels after 12 months. Another interim report from an inhalation study on rats exposed to 0, 20, or 80 ppm, 6 h per day, 5 days per week, showed tumors at the 80 ppm level after 12 months and on continued exposure, tumors were found in rats exposed at the 20 ppm level. (Ref. 5.6.)
Acrylonitrile poisoning in humans most frequently causes slight jaundice, gastritis, respiratory difficulties, and fatigue. Workers experienced respiratory and nervous system effects when exposed to acrylonitrile at 35-220 mg/m3 levels for 20-45 min. (Ref. 5.6)
- "Long-term exposure: Acrylonitrile has been shown to cause
cancer in laboratory animals and has been associated with higher
incidences of cancer in humans. Repeated or prolonged exposure of
the skin to acrylonitrile may produce irritation and dermatitis."
(Ref. 5.7.)
1.1.3 Potential workplace exposure
Acrylonitrile is used primarily in the manufacture of acrylic and
modacrylic fibers,
In 1976 there were 690 million kilograms of acrylonitrile produced in the United States. NIOSH estimates that there are 125,000 people potentially exposed in U.S. workplaces. (Ref. 5.6)
1.1.4 Physical properties (Ref. 5.7)
| boiling point range: | 77.5 - 79°C |
| melting point: | -83.5°C |
| flash point (open cup): | 0°C |
| vapor pressure (at 23°C): | 100 mm Hg |
| density (20/4): | 0.8060 |
| molecular weight: | 53.1 |
| synonyms: | 2-propenenitrile; cyanoethylene; vinyl cyanide; fumigrain; ventox; carbacryl; acrylon; AN. |
| molecular formula: | CH2CHCN |
1.2 Limit defining parameters (All of the acrylonitrile air concentrations listed in this procedure are based on a 20-L air sample and 1-mL desorption volume.)
- 1.2.1 Detection limit of the analytical procedure.
The detection limit of the analytical procedure is 0.36 ng per injection. This is the amount of analyte which will give a peak whose height is approximately 5 times the baseline noise. (Section 4.1.1)
1.2.2 Detection limit of the overall procedure.
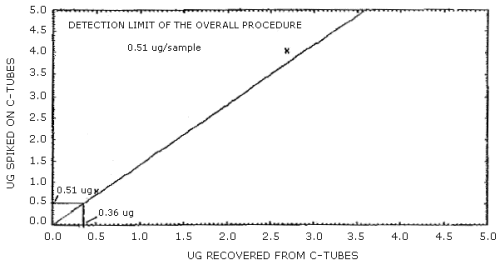
The detection limit of the overall procedure is 0.51 µg per sample (0.026 mg/m3 or 0.01 ppm). This is the amount of acrylonitrile spiked on a charcoal tube which allows recovery of an amount equivalent to the detection limit of the analytical procedure. (Section 4.1.2)
1.2.3 Reliable quantitation limit
The reliable quantitation limit is 13.2 µg per sample (0.66 mg/m3 or 0.3 ppm). This is the smallest amount of acrylonitrile which can be quantitated within the requirements of 75% recovery and 95% confidence limits of ±25%. (Section 4.2)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4 Sensitivity
The sensitivity of the analytical procedure over a range representing 0.5 to 2 times the target concentration is 1159 area counts/(µg/mL). This is determined from the slope of the calibration curve. (Section 4.4) The sensitivity will vary with the instrumentation used.
1.2.5 Recovery
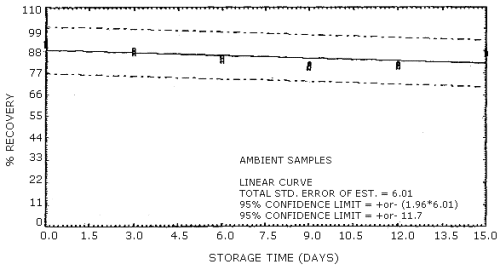
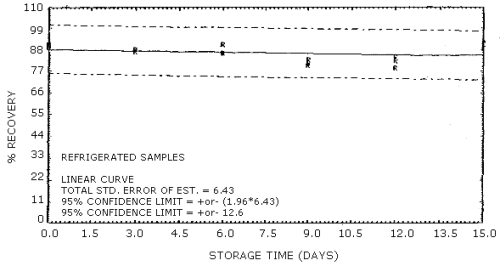
The recovery of acrylonitrile from the charcoal tubes during storage must be 75% or greater. The recovery from samples used in a 15-day storage test remained above 81% when the samples were stored at ambient temperature. (Section 4.7)
1.2.6 Precision (analytical procedure only)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1, and 2 times the target concentration is 0.0051. (Section 4.3.1)
1.2.7 Precision (overall procedure)
The overall procedure must provide results at the target
concentration that are ±25% or better at the 95% confidence level.
The precision at the 95% confidence level for the
1.2.8 Reproducibility
Six charcoal tubes were spiked with known amounts of acrylonitrile and analyzed immediately by a chemist unassociated with this procedure. The average recovery was 98% with a standard deviation of 3.4%. (Section 4.8)
1.3 Advantages
- 1.3.1 Charcoal tubes are readily available.
1.3.2 The samples may be analyzed more than once if necessary.
1.3.3 The method is rapid and precise.
1.3.4 The NPD is selective and sensitive.
1.4 Disadvantages
- 1.4.1 Acrylonitrile does not have constant desorption at
loadings below 40 µg for the 100-mg charcoal section and 16 µg for
the 50-mg section. This requires special consideration. (Section
3.7.4)
1.4.2 The method has not been field tested.
2. Sampling Procedure
- 2.1 Apparatus
- 2.1.1 A personal sampling pump which may be calibrated within ±5%
of the recommended flow rate should be used.
2.1.2 Coconut shell charcoal adsorbent tubes, 7-cm length, 6-mm o.d. and 4-mm i.d. containing a 100-mg front section and a 50-mg back section. For this evaluation, SKC lot 120 charcoal tubes were used.
2.2 Reagents
None required
2.3 Technique
- 2.3.1 Immediately before sampling, break open the ends of the
tubes. All tubes must be from the same lot of charcoal.
2.3.2 Connect the tube to the sampling pump with a short piece of tubing. The 50-mg portion of the charcoal tube is used as a backup section, therefore, air should pass through it after the air has passed through the 100-mg section.
2.3.3 Position the tube vertically near the breathing zone of the worker.
2.3.4 Air being sampled must not pass through any hose or tubing before entering the 100-mg section of charcoal.
2.3.5 Seal the ends of the tubes with plastic caps immediately after sampling and then wrap with OSHA label 21.
2.3.6 Submit at least one blank charcoal tube with each set of samples. Handle the blank tubes in the same manner as the samples (break ends, seal, transport), but do not draw air through the blanks.
2.3.7 Transport the samples and corresponding paperwork to the laboratory for analysis.
2.3.8 Submit bulk samples when necessary. Place bulk samples in glass bottles with Teflon-lined caps and mail in packages which do not contain air samples.
2.4 Breakthrough
- 2.4.1 For this evaluation, duplicate breakthrough studies at
approximately 8 mg/m3 and 80% relative
humidity (RH) were performed using a sampling rate of 0.2 L/min. The
average air volume sampled before 5% breakthrough occurred was 36.7 L
and the average loading on the 100-mg portion of charcoal was 0.295
mg. (Section 4.5.1)
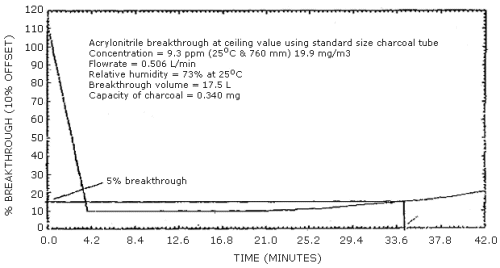
2.4.2 Several 5% breakthrough studies were also performed at the ceiling concentration of 10 ppm (21.7 mg/m3) to determine the maximum sampling flow rate that could be used to maximize the volume of air collected in a 15-min sampling period. It was found that 5% breakthrough occurred at an average of 35 min when a flow rate 0.5 L/min was used. (Figure 4.5.2. Ceiling breakthrough studies performed April 1983.)
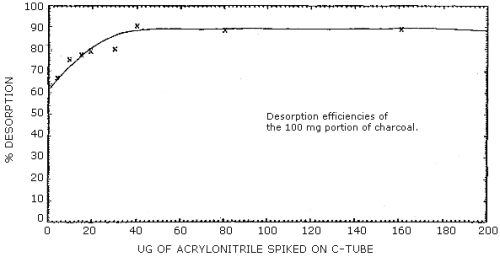
2.5 Desorption efficiency
- 2.5.1 The average desorption efficiency of acrylonitrile from the
100-mg portion of charcoal liquid spiked at loadings above 40 µg (0.5×
target concentration) was 89%. At loadings lower than 40 µg, the
desorption efficiency becomes dependent on the loading of
acrylonitrile and additional calculations (Section 3.7.4.) must be
performed. (Section 4.6.1)
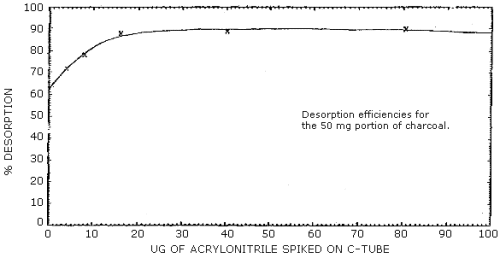
2.5.2 The desorption efficiency of the 50-mg portion of charcoal was constant above a loading of 16 µg. This average desorption efficiency was 89%. At loadings lower than 16 µg the desorption efficiency is not constant and additional calculations (Section 3.7.4) must be performed. (Section 4.6.2)
2.6 Recommended air volume and sampling rate
- 2.6.1 The recommended air volume is 20 L taken at a flow rate 0.2
L/min.
2.6.2 The recommended sampling conditions for monitoring ceiling concentrations are a 15-min sample taken at a flow rate of 0.5 L/min. This provides a sample air volume of 7.5 L. (The ceiling PEL for acrylonitrile is 10 ppm.)
2.7 Interferences
- 2.7.1 Relative humidities greater than 80%, and temperatures
greater than 25°C, may cause a decrease in the capacity of the
charcoal to adsorb and retain acrylonitrile.
2.7.2 It is not known if other chemicals may cause interferences with the sampling procedure.
2.8 Safety precautions
- 2.8.1 Wear eye protection when breaking off the ends of the
charcoal tubes.
2.8.2 Position the sampling device on the worker so as not to interfere with work or safety.
2.8.3 Observe all safety regulations of the area in which sampling is performed.
3. Analytical Procedure
- 3.1 Apparatus
- 3.1.1 Gas Chromatograph equipped with a nitrogen/phosphorus
detector: For this evaluation, a Hewlett Packard 5730A GC with a
Hewlett Packard NPD was used.
3.1.2 A GC column capable of separating acrylonitrile from the solvent front, interferences listed, and the internal standard: For this evaluation a 20-ft × 1/8-in. stainless steel column packed with 10% SP-1000 on 80/100 Supelcoport was used.
3.1.3 A method of peak area integration: A Hewlett Packard model 3354 data system was used in the evaluation.
3.1.4 Two-milliliter vials with Teflon-lined caps were used for desorption.
3.2 Reagents
- 3.2.1 Acetone, reagent grade.
3.2.2
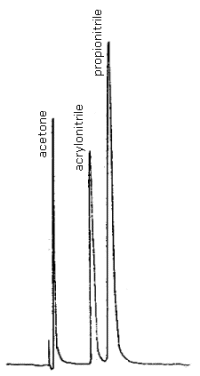
3.2.3 Internal Standard: Any compound may be used provided it does not adsorb onto charcoal from an acetone solution and it responds with an NPD. For this evaluation, propionitrile, reagent grade, was found suitable.
3.2.4 Hydrogen, GC grade
3.2.5 Air, GC grade.
3.2.6 Helium or nitrogen, GC grade.
3.2.7 Desorbing solution: Acetone containing 0.1 µL/mL propionitrile was used in this evaluation.
3.3 Standard preparation
Prepare standards with the desorbing solvent, either acetone alone or acetone containing an internal standard. Standards should be prepared in the range 0.1 µL/mL down to 0.01 µL/mL when sample air volumes are 20 L. Sample concentrations should be bracketed with standards.
3.4 Sample preparation
- 3.4.1 Place the 100-mg portion of charcoal in one vial and the
50-mg portion in a second vial. The glass wool and urethane plugs are
discarded.
3.4.2 Dispense 1.0 mL of the desorbing solvent into each vial containing charcoal.
3.4.3 Immediately cap the vials and allow them to desorb for 1 h with intermittent shaking.
3.5 Analysis
- 3.5.1 GC conditions
| temperatures | |
| oven: | 100°C |
| injector: | 200°C |
| detector: | 200°C |
| gases | |
| nitrogen: | 20 mL/min |
| hydrogen: | 3 mL/min |
| air: | 50 mL/min |
| detector voltage: | 19 |
| injection size: | 1 µL |
| retention time: | 5.35 min |
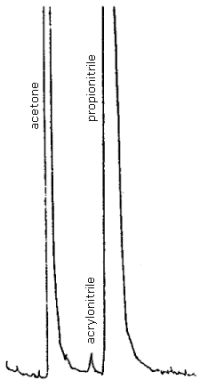
| chromatogram: | Figure 4.8 |
Update, April 1983: If problems with tailing peaks and poor separation of acrylonitrile and the internal standard occur with the above conditions, an alternate column which may be used is a 4-ft × 1/8-in. stainless steel column packed with Porapak QS. When this column is used the oven temperature should be 140°C.
3.5.2 An internal standard method is preferable since it corrects for injection size and slight changes in the NPD response.
3.5.3 A calibration curve is determined from the analytical standards by plotting detector response against the analytical standard concentrations (in µg/mL).
3.5.4 Samples are analyzed and the acrylonitrile peak areas are recorded.
3.6 Interferences
- 3.6.1 Any compound which has the same general retention time as
acrylonitrile or the internal standard on the column chosen and gives
a NPD response is an interference.
3.6.2 Interferences can frequently be alleviated by changing the GC conditions or column.
3.7 Calculations
- 3.7.1 The concentration, in µg/mL, of each desorbed sample is
determined from the calibration curve (Section 3.5.3)
3.7.2 The concentration for each sample is multiplied by the desorption volume, 1 mL, to get the total mass of acrylonitrile desorbed from the charcoal. This µg value is uncorrected for the blank sample and desorption efficiency.
3.7.3 A blank correction is made to the results in Section 3.7.2, if necessary.
3.7.4 The blank corrected mass of acrylonitrile found in each sample is corrected for incomplete desorption by use of the appropriate option: (The desorption efficiencies (DE) used in this method were obtained using SKC lot 120 charcoal and they should be redetermined when different charcoal is used.)
Option I
When the mass of acrylonitrile found (Section 3.7.3) for the 100-mg portion of charcoal is 36 µg or more and for the 50-mg portion of charcoal is 14 µg or more, the DE is constant (89%) and the mass should be divided by 0.89 to make the DE correction.
Option II
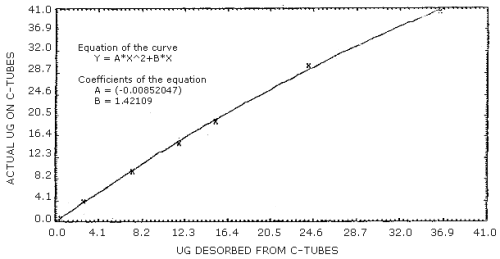
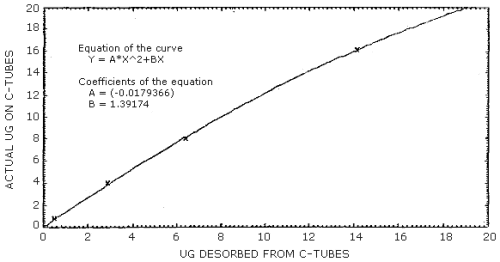
When the mass of acrylonitrile found (Section 3.7.3) is less than 36 µg for the 100-mg portion of charcoal or less than 14 µg for the 50-mg portion, the desorption efficiencies decrease with decreasing loadings (Table 4.6.1 and 4.6.2), and the correction for incomplete desorption must be determined with the use of the desorption curve prepared for each portion of charcoal. The desorption curve for each portion of charcoal was prepared by plotting analytical data obtained from spiked samples in the form of "actual µg" versus "µg desorbed". (Figure 4.9.1 and Figure 4.9.2) The equations of the curves (presented on the figures) may be used to calculate the mass corrected for incomplete desorption.
Example:
- Blank-corrected mass (100-mg portion) = 20 µg
| Y | = | AX2 + BX |
| Y | = | (-0.00852047)(20)2 + (1.42109)(20) |
| Y | = | 25.0 |
| mass, corrected for DE = 25 µg | ||
If the mass of acrylonitrile on either portion of charcoal is so small as to have a corresponding desorption efficiency of less than 75%, these values cannot be defended by this evaluation.
3.7.5 The air concentration (in mg/m3) of acrylonitrile is obtained by adding the mass from both portions of charcoal together and dividing by the air volume in L. The equivalent concentration in ppm at 760 mm Hg and 25°C may be obtained by multiplying the mg/m3 air concentration by the molar volume of an ideal gas, 24.46 L, and dividing by the molecular weight, 53.1.
3.7.6 When confirmation is required it should be obtained by GC/mass spectrometry or another suitable technique. Retention time on one column is not considered proof of identity.
3.8 Safety precautions
- 3.8.1 Work in a hood when preparing standards and samples.
3.8.2 Keep volumetrics and vials containing solvents away from sources of high temperature such as detectors and injectors.
3.8.3 Avoid skin contact with solvents.
3.8.4 Wear safety glasses at all times.
4. Backup Data
- 4.1 Detection limits
- 4.1.1 The detection limit of the analytical procedure was found
by injecting l µL of an analytical standard of 0.36 µg/mL (0.36
µg/mL × 0.001 mL = 0.36 ng/injection). This gave a peak which was
approximately 5 times the baseline noise. (Figure 4.1.1)
4.1.2 The detection limit of the overall procedure is 0.51 µg/sample (0.026 mg/m3 or 0.01 ppm). This is the amount of acrylonitrile which when spiked on a charcoal tube will allow recovery of an amount equivalent to the detection limit of the analytical procedure, 0.36 ng/injection. This value, 0.51 µg/sample, was obtained by plotting the µg of acrylonitrile spiked onto charcoal tubes vs. the µg of acrylonitrile recovered. Then using this curve to extrapolate from 0.36 µg recovered to the Y-axis and the values of the µg spiked were read (Figure 4.1.2). The injection size recommended in the analytical procedure (1 µL) was used for the determination of this detection limit.
4.2 Reliable quantitation limit
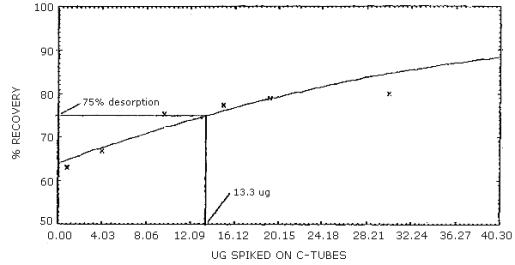
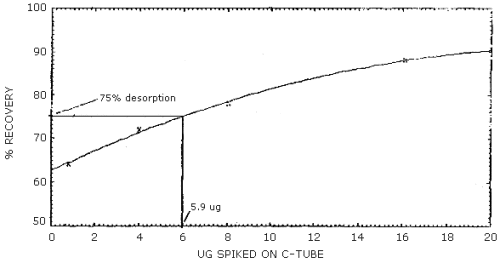
The reliable quantitation limit is that amount of acrylonitrile which when spiked onto the sampling device will allow recovery of at least 75% and have a precision (±1.96 SD) of ±25% or better. There are two reliable quantitation limits for this method; one for the 100-mg portion of a charcoal tube and another for the 50-mg portion. The value for the 100-mg portion is 13.3 µg and for the 50-mg portion is 5.9 µg. (Figures 4.2.1 and 4.2.2, respectively). This is due to differences in the desorption efficiencies of the different amounts of charcoal. (Figures 4.6.1 and 4.6.2) The injection size recommended in the analytical procedure (1 µL) was used for these determinations.
4.3 Precision data
- 4.3.1. The precision of the analytical procedure is defined as
the pooled coefficient of variation determined from replicate
injections of analytical standards at 0.5, 1, and 2 times the target
concentration. The pooled coefficient of variation is 0.0051. (Table
4.4.)
4.3.2. The precision of the overall procedure must be ±25% or better at the 95% confidence level for samples collected at the target concentration. The precision at the 95% confidence level for the 15-day storage test is ±12.6%. (Table 4.7., Figure 4.7.2.) This includes an additional ±5% for sampling error.
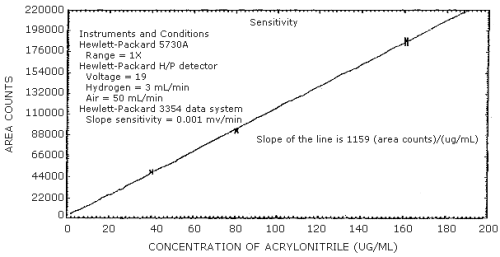
4.4 Sensitivity
The sensitivity is defined to be the slope of the calibration curve. (Table 4.4, Figure 4.4) The sensitivity is 1159 area counts per µg/mL.
Sensitivity and Precision Data
|
| |||
| × target conc. µ/mL |
0.5× 40.3 |
1× 80.6 |
2× 161.2 |
|
| |||
| area
counts SD CV |
49653 49730 49616 49708 49674 49579 49660 56.5 0.00114 |
92739 93212 92923 92626 92595 93064 92859 249 0.00268 |
184618 187038 188154 186809 189010 188409 187339 1573 0.00840 |
|
| |||
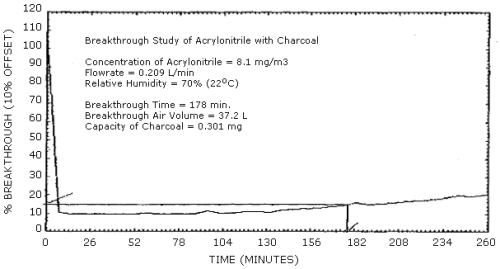
4.5 Breakthrough
- 4.5.1 Five percent breakthrough is defined for this method as
the point during sampling of an atmosphere at twice the target
concentration, using only the 100-mg portion of a charcoal tube,
when the acrylonitrile concentration passing through the charcoal
tube is 5% of the concentration of the atmosphere being sampled. At
a flow rate of 0.2 L/min with RH of 80% the average air volume
sampled before 5% breakthrough occurred was 36.7 L and the average
capacity of the charcoal tubes was 0.295 mg. (Table 4.5.1, Figure
4.5.1)
Breakthrough
|
| ||
| tube | air volume sampled | capacity |
|
| ||
| 1 2 |
37.2 L 36.2 L |
0.301 mg 0.289 mg |
|
| ||
4.5.2 Breakthrough studies were performed in a similar manner at the ceiling concentration PEL of 10 ppm. Five percent breakthrough occurred at approximately 35 min using a flow rate of 0.5 L/min. (Figure 4.5.2)
4.6 Desorption efficiencies
- 4.6.1. The DE for the 100-mg portion of charcoal was performed
by spiking the charcoal with microliter quantities of solutions
containing acrylonitrile in acetone. Six tubes were prepared at each
of several loadings, allowed to equilibrate overnight, then desorbed
with 1 mL of desorbing solution, and analyzed. The desorption
efficiencies at loadings equivalent to 0.5, 1, and 2 times the
target concentration were constant and averaged 89%. (Table 4.6.1.,
Figure 4.6.1.) The desorption efficiencies at lower loadings were
not constant. (Table 4.6.1., Figure 4.6.1.) Samples containing
loadings of acrylonitrile at these lower levels must have results
calculated according to Section 3.7.4.
Desorption Efficiencies From 100 mg Portions of Charcoal
|
| |||
| × target conc. µg/sample |
2× 161.2 |
1× 80.6 |
0.5× 40.3 |
|
| |||
| % DE (µg
found) |
91.1(146.8) 91.0(146.7) 86.8(139.9) 86.7(139.7) 89.4(144.0) 89.7(144.6) 89.1(143.6) |
88.5(71.36) 87.5(70.56) 91.0(73.33) 89.8(72.40) 88.0(70.95) 86.6(69.76) 88.6(71.39) |
91.0(36.68) 94.3(38.01) 87.5(35.35) 90.0(36.27) 88.7(35.73) 92.4(37.26) 90.6(36.55) |
|
| |||
| × target conc. µg/sample |
0.4× 30.2 |
0.24× 19.34 |
0.2× 15.1 |
|
| |||
| % DE (µg
found) |
80.2(24.23) 82.2(24.81) 80.9(24.44) 77.2(23.31) 79.3(23.96) 79.9(24.14) 80.0(24.15) |
80.4(15.54) 79.4(15.36) 77.9(15.06) 79.8(15.44) 77.1(14.91) 80.0(15.47) 79.1(15.29) |
78.8(11.90) lost 78.5(11.85) 76.4(11.53) 76.0(11.47) 77.4(11.69) 77.4(11.69) |
|
| |||
| × target conc. µg/sample |
0.1× 9.67 |
0.05× 4.03 |
0.01× 0.806 |
|
| |||
| % DE (µg
found) |
77.7(7.51) 76.1(7.36) 75.5(7.30) 74.0(7.16) 73.3(7.09) 75.4(7.30) 75.4(7.29) |
66.4(2.67) 67.7(2.73) 68.9(2.78) 66.1(2.66) 65.1(2.62) 66.8(2.69) 66.8(2.69) |
60.5(0.488) 66.6(0.537) 61.4(0.495) 66.0(0.0532) 62.0(0.506) 63.0(0.508) 63.2(0.511) |
|
| |||
4.6.2 Desorption efficiencies were performed using the 50-mg portion of charcoal. Again six tubes were spiked at each loading. Three of the tubes for each loading were desorbed with 0.5 mL and the other 3 with 1.0 mL to see if there was any relationship between the ratio of solvent to charcoal. There was no apparent trend with the amount of desorbing solvent added so all the data are presented together in Table 4.6.2. There was a constant desorption efficiency of 89% for loadings of acrylonitrile above 16 µg but at lower loadings the desorption varied. (Table 4.6.2 and Figure 4.6.2) Results for samples containing less than 16 µg on the 50-mg portion of charcoal must be calculated as described in Section 3.7.4.
Desorption Efficiencies From 50 µg Portions of Charcoal
|
| |||
| × target conc. µg/sample |
1× 80.6 |
0.5× 40.3 |
0.2× 16.1 |
|
| |||
| % DE (µg
found) |
85(68.51) 93(74.96) 89(71.73) 89(71.90) 93(74.98) 90(72.91) 90(72.50) |
88(35.6) 84(33.9) 87(35.1) 91(36.5) 89(35.6) 91(36.5) 88(35.6) |
87(14.06) 88(14.14) 87(13.55) lost 91(14.61) 89(14.39) 88(14.15) |
|
| |||
| × target conc. µg/sample |
0.1× 8.06 |
0.05× 4.03 |
0.01× 0.806 |
|
| |||
| % DE (µg
found) |
68(5.52) 78(6.26) 82(6.55) 79(6.44) 81(6.55) 77(6.15) 78(6.41) |
68(2.74) 72(2.90) 70(2.83) 77(3.09) 69(2.80) 74(3.00) 72(2.88) |
69(0.55) 67(0.54) 69(0.56) 52(0.42) 70(0.56) 59(0.48) 64(0.52) |
|
| |||
4.7 Storage data
Thirty-six charcoal tube samples were dynamically generated by
sampling an atmosphere at the target concentration with RH of 80%.
Twenty liters of air were drawn through each charcoal tube. Six of the
samples were analyzed immediately after collection. The remaining 30
samples were divided into two 15-sample sets. One set was stored in a
refrigerator
Storage Tests
|
| |||||||
| storage time (days) |
%
recovery (refrigerated) |
%
recovery (ambient) | |||||
|
| |||||||
| 0 3 6 9 12 15 |
89 88 91 81 84 91 |
90 88 87 83 82 89 |
91 88 86 80 79 92 |
91 87 83 81 81 86 |
91 87 84 79 80 86 |
92 88 83 82 82 87 | |
|
| |||||||
4.8 Reproducibility
Six charcoal tubes were spiked with acrylonitrile solutions. These tubes plus a copy of this procedure were given to a chemist unassociated with this method. The results are presented below.
Reproducibility
|
| |||||||
sample |
portion of charcoal |
amount spiked |
amount recovered |
% recovered | |||
|
| |||||||
| 1 2 3 4 5 6 |
100-mg 50-mg 100-mg 50-mg 100-mg 50-mg 100-mg 50-mg 100-mg 50-mg 100-mg 50-mg |
16 µg 8 µg 80 µg None 32 µg 8 µg 80 µg None 16 µg 8 µg 32 µg 8 µg |
15.8 µg 8.1 µg 77.6 µg None 30.2 µg 7.4 µg 77.4 µg None 16.0 µg 8.0 µg 30.2 µg 8.2 µg |
99 101 97 -- 94 93 97 -- 100 100 94 103 | |||
| |||||||
|
| |||||||
Nine samples which had been stored for 29 days at -5°C showed loss and migration of acrylonitrile with loss of precision. These data were not used in this evaluation since the storage period exceeded 15 days.
5. References
- 5.1 Campbell, D.N.; Moore, R.H.; Am. Ind. Hyg. Assoc. J.
1979, 40, pg. 904-9.
5.2 "NIOSH Manual of Sampling and Analytical Methods". D.G. Taylor (Manual Coordinator). 2nd ed., U.S. DHEW/PHS/CDC/NIOSH, Vol. 3, method S156, 1977.
5.3 Chambers, D.; Acrylonitrile (Organic Method Evaluation Branch, OSHA Laboratory, Salt Lake City, Utah). unpublished 1978.
5.4 Marano, R.S.; Levine, S.P.; Harvey, T.M.; Anal. Chem. 1978, 50, pg. 1948-1950.
5.5 "Documentation of Threshold Limit Values"; 4th ed: American Conference of Governmental Industrial Hygienists: Cincinnati, 1981.
5.6 "IARC Monograph on Some Monomers, Plastics and Synthetic
Elastomers and Acrolein"; International Agency for Research on
Cancer: Switzerland, 1979, vol. 19, pg.
5.7 "General Industry: OSHA Safety and Health Standards (29 CFR 1910)"; U.S. Department of Labor: Washington D.C., 1981, Section 1910.1045, pg. 839.
Addendum 1
Introduction
The desorption efficiency of acrylonitrile with acetone was non-linear. A study was performed to find a desorption solvent which could desorb at least 75% of the acrylonitrile over the range of 0.1× to 2× the PEL, using lot 2000 charcoal tubes from SKC. A mixed solvent of 95:5 methylene chloride:methyl alcohol (with 1 µL/mL n-hexyl alcohol internal standard) gave recoveries above 95% for all concentrations spiked. The sensitivity of an analysis performed using a capillary column is more than the sensitivity of an analysis using a packed column, therefore, it was possible to perform this desorption study using a flame ionization detector. For greater sensitivity, the nitrogen/phosphorus detector, specified in this method, should be used. The internal standard of n-hexyl alcohol cannot be used if the analysis is performed using a nitrogen/phophorous detector.
| Experimental | |
| Instrument: | HP5890 gas chromatograph with flame ionization detector |
| Column: | 60-m × 0.32-mm i.d. capillary coated with a 5.0-µm df DB-1 (J&W Scientific, Folsom, CA) |
| Injection size: | 1 µL (12:1 split) |
| zone temperatures: | 50°C (column), hold 8 min, ramp at 10°C/min to
180°C, hold 6 min 210°C (injector) 240°C (detector) |
| run time: | 27 min |
| column gas flow: | 2.8 mL/min (hydrogen) |
| septum purge: | 1.9 mL/min (hydrogen) |
| retention times: | 5.089 min (methyl alcohol) 10.036 min (acrylonitrile) 10.467 min (methylene chloride) 23.594 min (n-hexyl alcohol) |
| FID conditions: | hydrogen flow: 40 mL/min air flow: 450 mL/min makeup flow: 30 mL/min (nitrogen) |
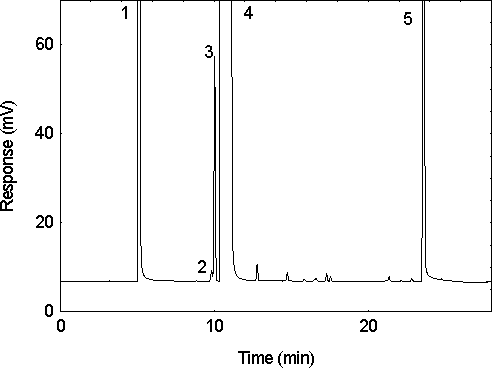
Figure 1. A chromatogram of an analytical standard of 40
µg/mL acrylonitrile in the solvent of 95:5 methylene chloride:methanol
with 1 µL/mL n-hexanol internal standard. (1 = methanol; 2 =
ethanol contamination in the methanol; 3 = acrylonitrile; 4 = methylene
chlorode; and 5 = n-hexyl alcohol).
Results
The desorption studies were performed by charcoal tubes, lot 2000, at concentrations of 2.001, 4.002, 20.001, 40.02, and 80.04 µg acrylonitrile, and allowing them to equilibrate overnight at room temperature. They were opened, each section of the tube placed into a separate labeled 2-mL vial, and desorbed with 1 mL of 95:5 methylene chloride:methyl alcohol with 1.0 µL/mL n-hexyl alcohol internal standard, and then placed on the shaker for 1 hour. They were analyzed by gas chromatography with a flame ionization detector (GC-FID). The detection limit was 1.0 µg.
| Desorption Efficiency of Acrylonitrile | |||||
|
% Recovered | |||||
| Tube # | 2.001 µg | 4.002 µg | 20.01 µg | 40.02 µg | 80.04 µg |
| 1 2 3 4 5 6 average overall ave |
95.1 96.4 97.1 94.3 95.1 94.7 95.5 98.0 |
98.5 96.8 97.8 97.0 97.6 98.0 97.6 |
98.3 96.8 98.4 99.6 99.1 98.9 98.5 |
100.2 98.9 99.7 99.1 99.9 99.1 99.5 |
99.3 97.9 99.7 98.6 97.8 98.9 98.7 |
Conclusion
Samples can be desorbed with a mixture of 95:5 methylene chloride:methyl alcohol with 1.0 µL/mL n-hexyl alcohol internal standard, and analyzed by GC-FID if the recommended air volume of 20 liters is collected. When lower air volumes are collected, the samples should be analyzed with a nitrogen/phosphorous detector after desorbing with the 95:5 methylene:methanol without the n-hexyl alcohol internal standard.