| Method no.: | 40 |
| Matrix: | Air |
| Target concentration: | 10 ppm (12.7 mg/m3) (OSHA PEL) |
| Procedure: | Samples are collected by drawing known volumes of air through standard size sampling tubes containing XAD-7 resin coated with 10% NBD chloride by weight. The samples are desorbed with 5% (w/v) NBD chloride in tetrahydrofuran (with a small amount of sodium bicarbonate present), heated in a hot water bath, and analyzed by high-performance liquid chromatography using a fluorescence or visible detector. |
| Recommended air volume and sampling rate: |
10 L at 0.2 L/min |
| Reliable quantitation limit: | 28 ppb (35 µg/m3) |
| Standard error of estimate at the target concentration: (Section 4.4.) |
5.8% |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: October 1982 | Chemist: Carl J. Elskamp |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General discussion
- 1.1. Background
- 1.1.1. History
The recommended air sampling procedure listed in the OSHA Field Operations Manual for methylamine is collection in midget impingers containing sulfuric acid (Ref. 5.1.). The analysis is done by gas chromatography. Impingers are cumbersome to use in the field and the analysis of free low molecular weight amines is difficult by gas chromatography (Ref. 5.2.). Thus better sampling and analytical procedures were needed for methylamine.
In NIOSH methods 221, 277, and S148 (Refs. 5.3. - 5.5.) silica gel is recommended for collection of methylamine in air. It was later found by NIOSH that low molecular weight amines are not stable after being collected on silica gel (Ref. 5.6.).
In this evaluation, it was found that methylamine could be
collected on a sampling tube containing XAD-7 resin coated with 10%
NBD chloride
1.1.2. Toxic effects (This section is quoted directly from "Occupational Health Guidelines for Chemical Hazards" (Ref. 5.9.) and is for information only and should not be taken as the basis of OSHA policy.)
- "Methylamine gas is a severe eye and respiratory irritant. The
LD50 was 0.1 to 0.2 g/kg in rats exposed
orally to a 40% aqueous solution of methylamine. One case of
bronchitis in a chemical worker has been reported; concentrations
measured in the workroom ranged from 2 to 60 ppm; the duration of
the exposure was not given. Brief exposures to 20 to 100 ppm are
said to produce transient irritation of the eyes, nose, and
throat. No symptoms of irritation are produced from longer
exposures at less than 10 ppm. One drop of 5% aqueous solution
caused conjunctival hemorrhage, superficial corneal opacities, and
edema in experimental animals; a 40% solution caused corneal
damage in rabbits. A 40% solution caused necrosis when applied to
the skin of a rabbit. Dermatitis and conjunctivitis are
occasionally observed in workers after prolonged exposure to the
vapor."
1.1.3. Potential workplace exposure
Following are some common operations in which exposure to methylamine may occur as reported in "Occupational Health Guidelines for Chemical Hazards." (Ref. 5.9.)
Methylamine is used:
- in production of insecticides, herbicides, fungicides,
surfactants, rocket fuels, explosives, pharmaceuticals,
photographic chemicals, dyes, textiles, dye assists, rubber and
anticorrosive chemicals.
as a polymerization inhibitor of hydrocarbons during distillation.
to prevent coagulation and webbing in natural and synthetic latex.
to prevent polymerization in paint removers.
1.1.4. Physical properties (Ref. 5.9.)
| molecular weight: | 31.1 |
| boiling point: | -6.32°C (760 mm Hg) |
| color: | colorless gas |
| specific gravity: | 0.656 (water = 1) |
| formula: | CH3NH2 |
| vapor pressure at 20°C: | not pertinent |
| flash point: | not applicable (gas) |
| odor: | ammonia-like |
| flammable limits in air, % by volume: |
lower: 5; upper: 21 |
| autoignition temperature: | 430°C |
| synonyms: | anhydrous methylamine, monomethylamine |
1.2. Limit defining parameters (The methylamine air concentrations listed throughout this method are based on an air volume of 10 L and a solvent desorption volume of 2 mL. Air concentrations given in ppm are referenced to 25°C and 760 mm Hg.)
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 1.9 ng per injection. This is the amount of methylamine which will give a peak whose height is approximately five times baseline noise. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 0.35 µg per sample (28 ppb or 35 µg/m3). This is the amount of methylamine spiked on the sampling device which allows recovery of an amount of methylamine equivalent to the detection limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 0.35 µg per sample (28 ppb or 35 µg/m3). This is the smallest amount of methylamine which can be quantitated within the requirements of a recovery of at least 75% and a precision (1.96 SD) of ±25% or better. (Section 4.2.)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Sensitivity
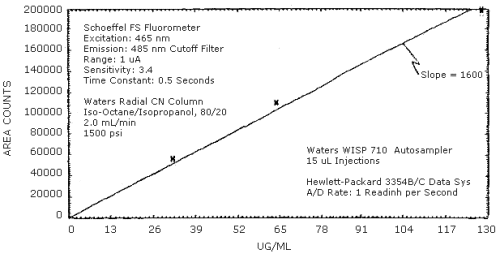
The sensitivity of the analytical procedure over a concentration range representing 0.5 to 2 times the target concentration is 1600 area units per µg methylamine/mL. This is determined by the slope of the calibration curve (Section 4.3.). The sensitivity will vary with the particular instrument used in the analysis.
1.2.5. Recovery
The recovery of methylamine from samples used in a 15-day storage test remained above 97% when the samples were stored at refrigerated or ambient temperature. (Section 4.4.) The recovery of methylamine from the collection medium during storage must be 75% or greater.
1.2.6. Precision (analytical method only)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1, and 2 times the target concentration is 0.009. (Section 4.3.)
1.2.7. Precision (overall procedure)
The precision at the 95% confidence level for a 15-day storage test is ±11.2%. This includes an additional ±5% for sampling error. (Section 4.4.) The overall procedure must provide results at the target concentration that are ±25% or better at the 95% confidence level.
1.2.8. Reproducibility
Six samples collected from a controlled test atmosphere, and a draft copy of this procedure were given to a chemist unassociated with this evaluation. The samples were analyzed after 14 days of storage at ambient conditions. The average recovery (corrected for desorption efficiency) was 98.3% with a standard deviation of ±1.7%. (Section 4.5.)
1.3. Advantages
- 1.3.1. The solid sorbent tube provides a convenient method for
sampling.
1.3.2. Methylamine is analyzed as a derivative which is specific, stable, and easier to quantitate than the free amine.
1.3.3. The analysis is rapid, sensitive, and precise.
1.3.4. This method is applicable to dimethylamine, ethylamine, and possibly other volatile aliphatic amines.
1.4. Disadvantages
- 1.4.1. The method has not been field tested.
1.4.2. Sampling tubes (XAD-7 coated with 10% NBD chloride) are not commercially available.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. Samples are collected by use of a personal sampling pump
that can be calibrated to within ±5% of the recommended flow rate
with the sampling tube in line.
2.1.2. Samples are collected on solid sorbent tubes containing XAD-7 coated with 10% NBD chloride by weight. The tube contains two sections of coated XAD-7 resin separated by a glass wool plug. The front section contains 80 mg of coated sorbent and the back section 40 mg. The sections are held in place with glass wool plugs in a glass tube 4-mm i.d. × 70-mm length.
The coated XAD-7 is prepared by rinsing the 20/50 mesh resin several times with methyl alcohol to remove fines. The resin is extracted for 24 h with methyl alcohol and dried by vacuum. The dried resin is then coated with 10% NBD chloride by weight using methylene chloride as a solvent. The solvent is removed by rotary evaporation.
2.2. Reagents
None required
2.3. Technique
- 2.3.1. Connect the sampling tube to the sampling pump with
flexible tubing. Air being sampled should not pass through any hose
or tubing before entering the sampling tube.
2.3.2. Place the sampling tube vertically in the employee's breathing zone.
2.3.3. After sampling, seal the tubes immediately with plastic caps and OSHA Form 21 seals.
2.3.4. Submit at least one blank for each sample set. The blank should be handled in the same manner as samples, except no air is drawn through it.
2.3.5. Record sample volume (in liters of air) for each sample, along with any potential interferences.
2.3.6. Ship any bulk sample(s) in a separate container(s) from the air samples.
2.4. Breakthrough
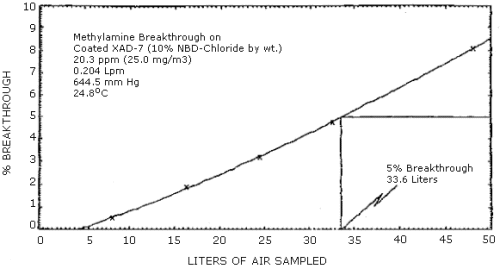
The average 5% breakthrough volume from a test atmosphere (air at approx. 80% relative humidity) containing 23.9 ppm (25.8 mg/m3) methylamine was determined to be 31.6 L. This corresponds to a loading of 0.815 mg of methylamine on the sampling tube. The sampling rate was approximately 0.2 L/min and the test atmosphere was at 24.8°C and 644.5 mm Hg. (Section 4.6.)
2.5. Desorption efficiency
- 2.5.1. The desorption efficiency of methylamine from spiked
samples was 94.3% over the range of 5 to 20 ppm. (Section 4.7.)
2.5.2. The desorption efficiency must be determined for each lot of coated XAD-7.
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 10 L.
2.6.2. The recommended sampling rate is 0.2 L/min.
2.7. Interferences (sampling)
- 2.7.1. An interference study was performed in which 10-L air
samples of a test atmosphere containing approximately 10 ppm each of
methylamine, ethylamine and dimethylamine were collected. The test
atmosphere was at approximately 80% relative humidity. There was no
difference in the amount of methylamine derivative recovered whether
the other amines were present or not.
2.7.2. It is not known if any compound(s) will interfere with the collection of methylamine on coated XAD-7 tubes.
2.7.3. Suspected interferences should be reported to the laboratory with submitted samples.
2.8. Safety precautions (sampling)
- 2.8.1. Attach the sampling equipment to the employee so that it
will not interfere with work performance or safety.
2.8.2. Follow all safety procedures that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. High-performance liquid chromatograph (HPLC) equipped
with a fluorescence and/or visible detector. For this evaluation a
Schoeffel FS 970 LC Fluorometer and a Waters 440 detector were used
in series with two Waters M-6000A pumps.
3.1.2. An HPLC column capable of separating the methylamine
derivative from NBD chloride and any interferences. A Radial CN
column was used in this study in the normal phase mode since the NBD
chloride derivatives fluoresce stronger in
3.1.3. An electronic integrator or some other suitable method of measuring peak areas or heights. A Hewlett-Packard 3354 B/C Data System was used in this evaluation.
3.1.4. A mechanical shaker.
3.1.5. A hot water bath.
3.1.6. Volumetric flask for preparing standards and making dilutions.
3.1.7. Pipets and syringes for preparing standards, making dilutions, and dispensing reagents.
3.1.8. Small vials with Teflon-lined caps capable of holding 3 mL.
3.2. Reagents
- 3.2.1. HPLC grade isopropanol and isooctane.
3.2.2. Reagent grade tetrahydrofuran (THF).
3.2.3. Reagent grade sodium bicarbonate.
3.2.4. Methylamine solution in water of known concentration or methylamine gas. A 40% solution from Fisher Scientific Company, Lot 712413 was used in this study.
3.2.5. Reagent grade NBD chloride. (7-chloro-4-nitrobenzo-2-oxa1,3-diazole). Aldrich Lot 1003 DH was used in this study.
3.2.6. Desorption reagent: 5 g of NBD chloride per 100 mL of THF.
3.3. Standard preparation
- 3.3.1. Prepare a stock standard of methylamine by diluting a
known volume of methylamine with THF. For this evaluation, a stock
standard of 16 µg/µL was prepared by diluting 2 mL of a 40% by
weight aqueous methylamine solution to 50 mL with THF.
3.3.2. Prepare working standards by injecting microliter amounts of the stock standard into 2.0 mL of desorption reagent in a small vial. Example: If a 5-µL aliquot of a stock standard at a concentration of 25 µg/µL is injected into a vial containing 2.0 mL of desorption reagent, the working standard is equal to 125 µg/sample (5 × 25) if the samples are desorbed with 2.0 mL. For a 10-L air sample this is equivalent to 12.5 mg/m3 (9.83 ppm), uncorrected for desorption efficiency.
3.3.3. Add approximately 25 mg of solid sodium bicarbonate to each vial and seal with Teflon-lined caps. (This is easily done by using the large end of a standard size disposable dropping pipette as a spatula). The standards are shaken for 0.5 h and then heated for 2.5 h at 60°C in a water bath. Allow standards to cool to room temperature before analyzing.
3.4. Sample preparation
- 3.4.1. Transfer each section of the sample to separate vials.
The glass wool plugs must be added to the vials if they contain
entrapped XAD-7 beads. The glass tube is discarded.
3.4.2. Add 2.0 mL of desorption reagent to each vial.
3.4.3. Add approximately 25 mg of solid sodium bicarbonate to each vial.
3.4.4. Seal the vials with Teflon-lined caps and shake in a horizontal position for 0.5 h. The vials should be positioned parallel to the shaker's movements.
3.4.5. Heat the vials for 2.5 h in a water bath at 60°C. Allow samples to cool to room temperature before analyzing.
3.5. Analysis
- 3.5.1. HPLC conditions
| fluorescence detector: | 465 nm excitation 525 nm emission |
| injection size: | 15 µL |
| column: | Waters Radial CN |
| solvent: | isooctane: isopropanol, 80:20 at 2 mL/min |
| retention time: | 3.75 min |
| alternate detector: | visible at 465 nm |
| chromatograms: | Section 4.8. |
3.5.2. Peak areas (or heights) are measured by an integrator or other suitable means.
3.5.3. A calibration curve is constructed by plotting peak areas (or heights) of standard injections versus µg methylamine per sample. Sample concentrations must be bracketed by standards.
3.6. Interferences (analytical)
- 3.6.1. Any compound that has the same general retention time as
the methylamine derivative and responds with the detector used is an
interference. Possible interferences should be reported to the
laboratory with submitted samples by the industrial hygienist. The
derivatives of ethylamine, dimethylamine, and diethylamine can be
separated from the methylamine derivative.
3.6.2. HPLC parameters (i.e. solvent composition, column, detector, etc.) may be changed to possibly circumvent interferences.
3.6.3. Retention time on a single column is not considered proof of chemical identity. Samples over the PEL should be confirmed by GC/MS or other suitable means.
3.7. Calculations
The methylamine concentration is obtained from the calibration curve in terms of micrograms per sample. The air concentration for samples is calculated using the following formulae. If any methylamine is found on the backup section, it is added to the amount found on the front section. This total amount is then corrected by subtracting the total amount found in the blank.
| mg/m3 = | (blank-corrected micrograms per
sample)
(liters of air sampled) (desorption efficiency) |
ppm = (mg/m3)(24.46)/(31.1) = (mg/m3)(0.7865)
| where | 24.46 | = | molar volume (liters) at 25°C, 760 mm Hg |
| 31.1 | = | MW of methylamine |
3.8. Safety precautions (analytical)
- 3.8.1. Avoid skin contact and inhalation of all chemicals used,
especially methylamine and NBD chloride.
3.8.2. Restrict the use of all chemicals to a fume hood if possible.
3.8.3. Wear safety glasses and a lab coat at all times while in the laboratory area.
4. Backup Data
- 4.1. Detection limit of the analytical procedure
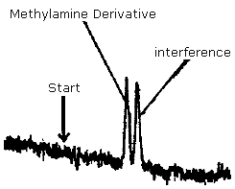
The detection limit of 1.9 ng per injection was determined by making a 15-µL injection of a 0.128 µg/mL standard. This standard is based on the weight of methylamine added to make the standard. Shown in Figure 4.1. is a chromatogram obtained from a Schoeffel FS 970 Fluorescence detector set at 0.1 µA range, 4.75 sensitivity and 0.5 second time constant. A Radial CN column was used. The recorder was set at 0.2 cm/min and 10 mV full scale. The solvent system used for this determination was 90:10, isooctane:isopropanol. This was used to obtain separation between the methylamine derivative and a small interference. This interference is insignificant for samples around the PEL. Regis NBD chloride was used in this determination since the interference peak was smaller than that found in Aldrich NBD chloride.
4.2. Detection limit of the overall procedure and reliable quantitation limit
Samples were prepared by spiking (liquid injection) the coated XAD-7 with 0.35 µg of methylamine. The samples were analyzed the next day. The amount recovered was essentially equivalent to the analytical detection limit of 0.26 µg per sample.
Detection Limit of the Overall Procedure
and Reliable Quantitation Limit Data
|
| |||
| % recovery | statistics | ||
|
| |||
| 75.9 75.9 73.9 79.8 71.6 75.9 |
SD |
= = |
75.5 2.7 |
|
| |||
4.3. Sensitivity and precision
The sensitivity and precision of the analytical procedure were determined from multiple injections of analytical standards. This data is given below and also shown graphically in Figure 4.3.
Sensitivity and Precision Data
|
| |||
| × target conc. µg/mL |
0.5× 32 |
1× 64 |
2× 128 |
|
| |||
| area
counts SD CV |
57004.2 56566.0 55669.2 56486.7 55630.7 56274.1 56271.8 537.3 0.0095 |
110045 108902 109441 109686 109902 109549 109587.5 402.7 0.0037 |
200318 200370 202752 199014 195434 198402 199381.7 2445.1 0.0123 |
|
| |||
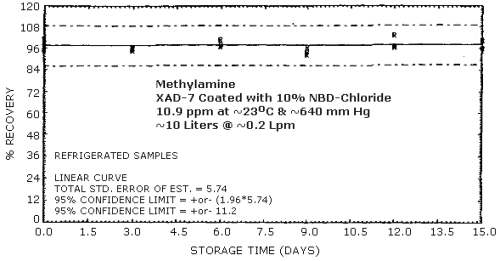
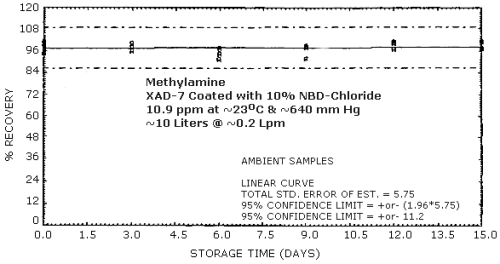
4.4. Recovery and storage
Storage samples were generated from a test atmosphere (air) containing 10.9 ppm methylamine at approximately 80% relative humidity, 23°C, and 640 mm Hg. Each sample was generated by sampling the test atmosphere at approximately 0.2 L/min for 50 min, resulting in a sample volume of about 10 L. An amount of coated XAD-7 equivalent to the front section of a standard adsorbent tube (about 80 mg) was used for each sample. After sampling, the adsorbent was transferred to separate vials, capped, and stored. Six samples were analyzed immediately after generation, 15 were stored in a closed drawer at ambient temperature, and 15 were stored under refrigeration at 0°C.
Storage Tests
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 0 3 6 9 12 15 |
101.1 99.4 94.4 100.9 97.1 103.8 100.6 |
99.5 97.0 95.8 97.0 94.0 97.6 96.3 |
94.8 98.7 96.6 97.9 92.4 96.9 100.8 |
101.1 99.4 100.3 97.7 99.2 101.4 101.4 |
99.5 97.0 96.2 94.6 92.2 97.2 97.4 |
94.8 98.7 98.2 91.2 98.0 100.4 97.6 | |
|
| |||||||
The data presented in Table 4.4. are shown graphically in Figures 4.4.1. and 4.4.2.
4.5. Reproducibility
Six methylamine samples were prepared with the vapor generator by sampling an air stream containing 10.9 ppm methylamine for 50 min at approximately 0.2 L/min. The sample stream was at 24°C, 638 mm Hg, and approximately 80% relative humidity. The samples were stored for 14 days at room temperature before being analyzed.
Reproducibility
|
| ||||||
| sample no. | mg found | mg expected | % found | |||
|
| ||||||
| 1 2 3 4 5 6 7 (blank) |
0.1486 0.1548 0.1419 0.1437 0.1406 0.1427 N.D. |
0.1468 0.1593 0.1457 0.01465 0.1442 0.1551 0.00 |
101.2 97.2 97.4 98.1 97.5 (92.0)1 --- | |||
|
||||||
|
| ||||||
| 1 This sample was not used for calculation of average % found. The orifice plugged during sampling, resulting in a lower sampling rate. | ||||||
4.6. Breakthrough
The breakthrough volume was determined from a test atmosphere containing 23.9 ppm (25.8 mg/m3) methylamine. The sampling tube contained only the front section (approximately 80 mg) of adsorbent. A backup tube was connected downstream from the sampling tube. This backup tube was changed periodically and analyzed to determine the amount of methylamine breaking through the sampling tube. The average breakthrough volume for three separate determinations was 31.6 L. This corresponds to an average loading of 0.814 mg methylamine on the sampling tube when 5% breakthrough occurred. The test atmosphere was at 24.8°C, 644.5 mm Hg, and approximately 80% relative humidity. A breakthrough curve for one of the tests is shown in Figure 4.6.
4.7. Desorption efficiency
The desorption efficiency was determined by injecting known amounts of a methylamine standard onto coated XAD-7 and analyzing the samples the next day.
Desorption Efficiency
|
| |||
| × target conc. µg/sample |
0.5× 64.0 |
1× 128 |
2× 256 |
|
| |||
| desorption efficiency, % |
94.7 96.9 87.0 100.0 96.4 100.5 95.9 |
93.1 93.0 92.3 97.1 96.4 94.1 94.3 |
92.3 94.1 92.2 93.1 89.5 94.8 92.7 |
|
| |||
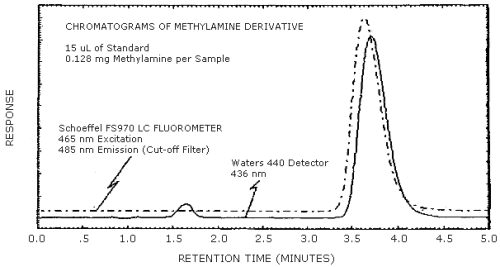
4.8. Chromatograms
Chromatograms of a methylamine standard are shown in Figure 4.8. The chromatograms are from a 15-µL injection of a 0.128 mg methylamine per sample standard. The fluorescence and visible detectors were connected in series to give essentially simultaneous chromatograms. The responses shown are standardized to keep the peaks about 90% full scale. Thus, this figure does not indicate the relative response of each detector.
5. References
- 5.1. "Industrial Hygiene Field Operation Manual", OSHA Instruction
CPL 2-2.20, Office of Field Coordination, 1979.
5.2. Dalene, M.; Mathiasson, L.; Jonsson, J.A. J. Chromatogr. (1981), 207, 37-46.
5.3. "NIOSH Manual of Analytical Methods", 2nd ed.; Department of Health, Education and Welfare, National Institute for Occupational Safety and Health: Cincinnati, OH 1977; Vol. 1, Method No. P&CAM 221; DHEW (NIOSH) Publ. (U.S.), No. 77-157-A.
5.4. "NIOSH Manual of Analytical Methods", 2nd ed.; Department of Health, Education and Welfare, National Institute for Occupational Safety and Health: Cincinnati, OH, 1978; Vol. 4, Method No. P&CAM 277; DHEW (NIOSH) Publ. (U.S.), No. 78-175.
5.5. "NIOSH Manual of Analytical Methods", Department of Health, Education and Welfare, National Institute for Occupational Safety and Health: Cincinnati, OH, 1980; Vol. 6, Method No. S148; DHHS (NIOSH) Publ. (U.S.), No. 80-125.
5.6. Teass, A. National Institute for Occupational Safety and Health, personal communication, 1981.
5.7. Elskamp, C.J., Dimethylamine (Method 34, Organic Methods Evaluation Branch, OSHA Analytical Laboratory, Salt Lake City, Utah). Unpublished (2-82).
5.8. Elskamp, C.J., Ethylamine (Method 36, Organic Methods Evaluation Branch, OSHA Analytical Laboratory, Salt Lake City, Utah). Unpublished (5-82).
5.9. "Occupational Health Guidelines for Chemical Hazards" NIOSH/OSHA, Jan. 1981, DHHS (NIOSH) Publication No. 81-123.