2-NITROPROPANE
| Method no.: | 46 |
| Matrix: | Air |
| Target concentration: | 25 ppm (91 mg/m3) (OSHA PEL for both analytes) |
| Procedure: | Samples are collected by drawing known volumes of air
through commercially available |
| Recommended air volume and sampling rate: |
4 L at 0.1 L/min |
| Reliable quantitation limit: | 25 ppb (91 µg/m3) for both analytes |
| Standard errors of estimate at the target concentration: (Section 4.4) |
1-Nitropropane, 7.2%; 2-Nitropropane, 6.2% |
| Special requirements: | After samples are received at the laboratory, they should be stored under refrigeration, until analyzed, to help minimize migration. |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: January 1984 | Chemist: Carl J. Elskamp |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
In the past there were no validated sampling and analytical
procedures for determining
Since there is a validated sampling and analytical procedure for
Breakthrough studies were performed with
Desorption efficiencies were determined for both
The collection of
1.1.2. Toxic effects. (The section for
- 1.1.2.1. 1-Nitropropane
"1-Nitropropane vapor is an eye irritant and in animals causes mild respiratory irritation and severe liver damage. Rabbits died from exposure to 5,000 ppm for 3 h, but 10,000 ppm for 1 h was not lethal. Effects were conjunctival irritation, lacrimation, slow respiration with some rales, muscular incoordination, ataxia, and weakness. Autopsy of animals exposed to lethal concentrations revealed severe fatty infiltration of the liver and moderate kidney damage. Human volunteers exposed to over 100 ppm noted eye irritation. There are no reports of systemic effects in humans."
1.1.2.2. 2-Nitropropane
"Exposure to concentrations of
1.1.3. Potential workplace exposure
Following are some common operations where exposure to
- 1.1.3.1. 1-Nitropropane
1-Nitropropane is used:
- as a thinner and solvent for cellulose compounds, lacquers,
and dopes; in vinyl resins for industrial coatings and printing
inks; in synthetic finish removers; and for oil and
spirit-soluble dyes of molded plastics.
as an extraction solvent for purification, separation, recrystallization, and recovery for natural and synthetic resins, tars, coating materials, fats, and oils.
a reaction medium in polymer technology, as a catalyst, initiator, and solvent.
in organic chemical synthesis for preparation of amines, nitrated alcohols, acids, and chloronitroparaffins.
in manufacture of explosives.
1.1.3.2. 2-Nitropropane
"Solvent systems containing
1.1.4. Physical properties (Ref. 5.3. unless otherwise noted)
| molecular weight: | 89.09 | 89.09 |
| boiling pt., 760 mm Hg: | 131.6°C | 120.3°C |
| color: | colorless | colorless |
| density (25/4°C): | 0.99 | 0.9821 |
| vapor pressure, 20°C: | 7.5 mm (Ref. 5.2.) | 12.9 (Ref. 5.1.) |
| flash pt., closed cup: | 34°C | 24°C |
| odor: | mild, fruity odor (Ref. 5.2.) |
|
| flammable limits in air, % by volume (lower): |
2.2 (Ref. 5.2.) |
2.6 (Ref. 5.1.) |
| autoignition temp: | 420.6°C (Ref. 5.2.) | |
| formula: | CH3CH |
CH3CH(NO2)CH3 |
| synonyms: | none (Ref. 5.2.) | dimethylnitro-methane; isonitropropane;
nitroisopropane and |
1.2. Limit defining parameters (The analyte air concentrations
listed throughout this method are based on an air volume of 4 L and
solvent desorption volume of 1.0 mL. The ppb and ppm values are
referenced to an atmospheric pressure of 760 mm Hg and temperature of
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 0.4 ng for
both
1.2.2. The detection limit of the overall procedure is 0.4 µg per
sample (25 ppb or 91 µg/m3) for both
1.2.3. The reliable quantitation limit is 0.4 µg per sample (25
ppb or 91 µg/m3) for both
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Sensitivity
The sensitivities of the analytical procedure over a
concentration range representing 0.5 to 2 times the target
concentration based on the recommended air volume are 420 area
counts per µg/mL for
1.2.5. Recovery
The recoveries of
1.2.6. Precision
The pooled coefficients of variation obtained from replicate
determinations of analytical standards at 0.5, 1, and 2 times the
target concentration are 0.009 for
1.2.7. Precision (overall procedure)
The precisions at the 95% confidence level for 15-day storage
tests are ±14.1% for
1.2.8. Reproducibility
Six samples for each analyte, collected from controlled test
atmospheres, and a draft copy of this procedure were given to a
chemist unassociated with this evaluation. The samples were analyzed
after 57 days of storage at 2°C. The average recoveries were 95.4%
and 97.6% with standard deviations of 1.2% and 2.4% for
1.3. Advantages
- 1.3.1. The solid sorbent tube provides a convenient method for
sampling.
1.3.2. The
1.3.3. The analysis is rapid, sensitive, and precise.
1.3.4. The desorption solvent is carbon disulfide, which is a better solvent for use with a flame ionization detector than ethyl acetate.
1.4. Disadvantages
- 1.4.1. This method has not been field tested.
1.4.2. The amount of sample that can be taken is limited by the
total milligrams the
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. Samples are collected by use of a personal sampling pump
that can be calibrated within ±5% of the recommended flow rate with
the sampling tube in line.
2.1.2. Samples are collected on solid sorbent sampling tubes
containing
2.2. Reagents
No sampling reagents are required.
2.3. Sampling technique
- 2.3.1. Immediately before sampling, break open the ends of the
2.3.2. Connect the sampling tube to the sampling pump with flexible tubing. Position the tube so that sampled air first passes through the 80-mg section.
2.3.3. Air being sampled should not pass through any hose or tubing before entering the sampling tube.
2.3.4. Place the sampling tube vertically (to avoid channeling) in the employee's breathing zone.
2.3.5. After sampling, seal the tubes immediately with plastic caps and wrap lengthwise with OSHA Form 21.
2.3.6. Submit at least one blank sampling tube with each sample set. Blanks should be handled in the same manner as samples, except no air is drawn through them.
2.3.7. Record sample volumes (in liters of air) for each sample, along with any potential interferences.
2.3.8. Ship any bulk sample(s) in a separate container(s) from the air samples.
2.4. Breakthrough
- 2.4.1. The average 5% breakthrough volume from a test atmosphere
(air at approx. 80% relative humidity) containing 52.6 ppm (191.8
mg/m3)
2.4.2. Since it was found that the breakthrough volume was
greater (as expected) for
2.5. Desorption efficiency
- 2.5.1. The average desorption efficiency of
2.5.2. The average desorption efficiency of
2.5.3. Desorption efficiencies must by determined for each lot of
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 4 L.
2.6.2. The recommended sampling rate is 0.1 L/min.
2.7. Interferences (sampling)
- 2.7.1. It is not known if any compound will severely interfere
with the collection of
2.7.2. Suspected interferences should be reported to the laboratory with submitted samples.
2.8. Safety precautions
- 2.8.1. Attach the sampling equipment to the employee so that it
will not interfere with work performance or safety.
2.8.2. Wear eye protection when breaking the ends of the
2.8.3. Follow all safety procedures that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A GC equipped with a flame ionization detector. For this
evaluation, a
3.1.2. A GC column capable of separating
3.1.3. An electronic integrator or some other suitable method of measuring peak areas or heights.
3.1.4. Small vials with Teflon-lined caps capable of holding 2 mL.
3.1.5. A dispenser capable of delivering 1.0 mL to prepare standards and samples. If a dispenser is not available, a 1-mL pipet may be used.
3.1.6. Syringes, such as 10-µL for preparation of standards and
3.1.7. Volumetric flasks and pipets to dilute the
3.2. Reagents
- 3.2.1. Carbon disulfide, reagent grade.
3.2.2. 1-Nitropropane and
3.2.3. GC grade nitrogen, air, and hydrogen.
3.3. Standard preparation
- 3.3.1. Analytical standards are prepared in carbon disulfide.
Dispense 1.0 mL of carbon disulfide (using the same dispenser or
pipet used for samples) into 2-mL vials. Seal the vials immediately
with Teflon-lined caps. Using a 10-µL syringe, dispense into the
sealed vials a known amount of a
3.3.2. Standard concentrations should bracket sample concentrations. Thus, if samples fall out of the concentration range of prepared standards, additional standards may have to be prepared and analyzed to ascertain linearity of response.
3.4. Sample preparation
- 3.4.1. Transfer each
3.4.2. Add 1.0 mL of carbon disulfide to each vial using the same dispenser as used for preparation of standards.
3.4.3. The vials are immediately capped and shaken periodically for 30 min before analysis.
3.5. Analysis
| GC conditions | |
| column: | 10-ft × 1/8-in SS, 20% SP-2100, 0.1% CW1500 on 100/120 Supelcoport |
| injection volume: | 1 µL |
| zone temperatures (°C): | 75 (column) 175 (injector) 250 (FID detector) |
| gas flows (mL/min): | 25 (nitrogen, carrier) 45 (hydrogen) 260 (air) |
| retention times (min): | 2.4 (carbon disulfide) 5.7 ( 7.3 ( |
| chromatograms: | Section 4.8. |
3.6. Interferences (analytical)
- 3.6.1. Any compound that responds on a flame ionization detector
and has the same general retention time of the analyte is a
potential interference. Possible interferences should be reported to
the laboratory with submitted samples by the industrial hygienist.
These interferences should be considered before samples are
desorbed.
3.6.2. GC parameters (i.e. column and column temperature) may be changed to possibly circumvent interferences.
3.6.3. Retention time on a single column is not considered proof of chemical identity. Samples should be confirmed by GC/MS if possible.
3.7. Calculations
The analyte concentration for samples is obtained from the appropriate calibration curve in terms of micrograms per sample, uncorrected for desorption efficiency. The air concentration is calculated using the following formulae. If any analyte is found on the backup section, it is added to the amount found on the front section. This total amount is then corrected by subtracting the total amount (if any) found in the blank.
| mg/m3 = | (blank corrected micrograms per
sample)
(liters of air sampled) (desorption efficiency) |
ppm = (mg/m3)(24.46)/(89.09) = (mg/m3)(0.2746)
| where | 24.46 = molar volume (liters) at 760 mm Hg,
25°C 89.09 = molecular weight of 1- and |
3.8. Safety precautions (analytical)
- 3.8.1. Avoid skin contact and inhalation of all chemicals.
3.8.2. Restrict the use of all chemicals to a fume hood when possible.
3.8.3. Wear safety glasses and a lab coat at all times while in the lab area.
4. Backup Data
- 4.1. Detection limit of the analytical procedure
The recommended injection size of 1 µL was used to determine the
detection limits of the analytical procedure. The detection limit of
0.4 ng for
4.2. Detection limit of the overall procedure and reliable quantitation limit data
Samples were prepared by injecting 368 ng of
Detection Limits of the Overall
Procedure and Reliable Quantitation Limits Data
|
| ||||
| 1-NP | 2-NP | 1-NP | 2-NP | |
| sample no. | mass spiked, ng | % recovery | ||
|
| ||||
| 1 2 3 4 5 6 SD 1.96 SD |
368 368 368 368 368 368 |
363 363 363 363 363 363 |
86.4 87.4 102.4 93.7 95.1 97.9 93.8 6.1 12.0 |
92.5 94.1 98.8 98.0 98.0 96.5 96.4 2.6 5.1 |
|
| ||||
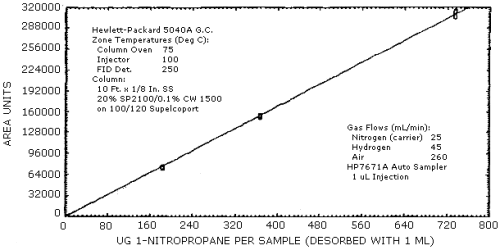
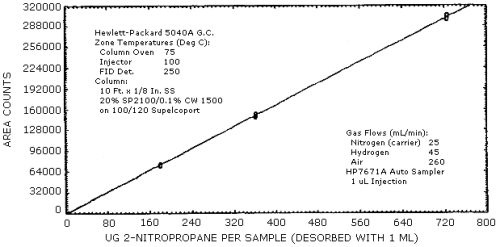
4.3. Sensitivity and Precision (analytical)
The sensitivity and precision of the analytical procedure were determined from multiple injections of analytical standards. These data are given in Table 4.3. and shown graghically Figure 4.3.1. and 4.3.2.
Sensitivity and Precision Data
|
| ||||||
| 1-NP | 2-NP | |||||
|
|
| |||||
| × target conc. µg/mL |
0.5× 183.8 |
1× 367.6 |
2× 735.1 |
0.5× 181.7 |
1× 363.4 |
2× 726.8 |
|
| ||||||
| area
counts SD CV(%) |
76240 74520 75100 74820 75280 75300 75210 585.5 0.78 |
152300 153500 152700 154400 152600 154900 153400 1058 0.69 0.009 |
306300 312000 306100 312900 306000 313900 309533 3774 1.22 |
73180 75080 74120 75280 73480 75380 74420 960 1.29 |
148800 151800 147600 152100 147900 152300 150083 2214 1.48 0.013 |
298600 305000 298800 305600 299100 306100 302200 3708 1.23 |
|
| ||||||
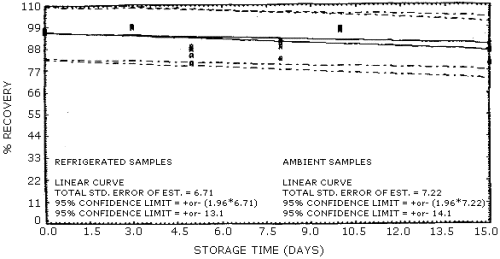
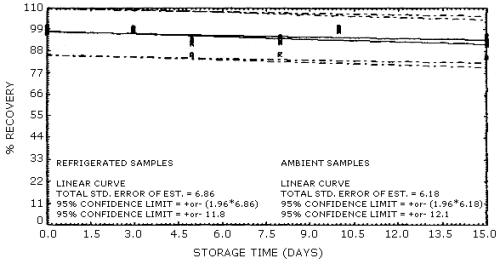
4.4. Recovery data (storage)
Storage samples were generated from test atmospheres (air at about
80% relative humidity) containing
Storage Tests for 1-Nitropropane
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 0 3 5 8 10 15 |
97.7 96.7 99.0 89.3 89.0 98.0 90.6 |
96.6 96.8 99.2 87.8 92.1 98.2 90.6 |
97.1 96.3 99.0 87.9 83.2 98.7 90.6 |
97.7 96.7 98.6 84.8 91.5 97.1 87.1 |
96.6 96.8 98.9 88.1 90.4 97.7 87.7 |
97.1 96.3 99.2 80.7 90.3 97.6 81.0 | |
|
| |||||||
Storage Tests for 2-Nitropropane
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 0 3 5 8 10 15 |
98.0 100.5 100.0 94.2 92.8 99.2 96.0 |
97.4 97.7 99.5 92.2 84.0 99.4 93.9 |
97.4 98.3 98.2 92.7 86.5 100.4 93.1 |
98.0 100.5 99.2 93.6 95.7 99.6 91.6 |
97.4 97.7 98.4 93.8 93.9 98.6 94.0 |
97.7 98.3 100.1 86.4 92.9 98.1 85.3 | |
|
| |||||||
4.5. Reproducibility
Six samples for each analyte, collected from controlled test atmospheres (80% RH, 25°C, 656 mm Hg) containing the analyte near the target concentration, were analyzed by another chemist unassociated with this evaluation. The samples were generated by drawing the test atmosphere through the sampling tubes for 40 min at approximately 0.1 L/min. The samples were stored for 57 days at 2°C before being analyzed. The results are given in Table 4.5.
Reproducibility
|
| ||||||
| 1-NP | 2-NP | |||||
|
|
| |||||
sample |
µg found |
µg possible |
% found |
µg found |
µg possible |
% found |
|
| ||||||
| 1 2 3 4 5 6 SD |
333.3 394.3 350.1 363.6 402.3 |
342.8 412.3 372.8 380.3 423.7 (sample lost) |
97.2 95.6 93.9 95.6 94.9 95.4 1.2 |
340.7 422.8 379.0 384.1 410.7 432.4 |
358.2 423.8 385.8 394.1 434.1 429.3 |
95.1 99.8 98.2 97.5 94.6 100.7 97.6 2.4 |
|
| ||||||
4.6. Breakthrough
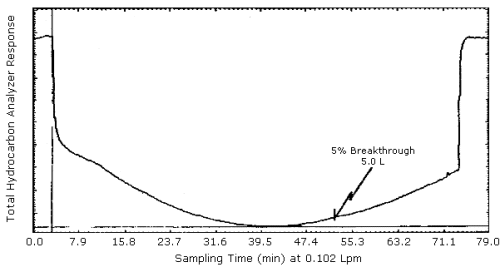
The average 5% breakthrough volume of 4.8 L for
4.7. Desorption efficiency
The desorption efficiency for each analyte was determined by
injecting known amounts of
Desorption Efficiency Data
|
| ||||||
| 1-NP | 2-NP | |||||
|
|
| |||||
| µg ppm |
183.8 12.6 |
367.6 25.2 |
735.1 50.4 |
181.7 12.5 |
363.4 24.9 |
726.8 49.9 |
|
| ||||||
| % desorption |
95.1 95.3 95.4 94.5 95.9 94.5 95.1 |
95.0 95.7 95.6 95.0 96.1 94.1 95.2 95.4 |
95.3 94.7 95.6 96.7 96.2 96.0 95.8 |
99.6 98.3 98.0 98.0 94.0 98.1 97.7 |
98.3 96.7 96.3 95.7 96.7 96.2 96.7 96.4 |
93.8 95.1 95.3 95.0 94.6 95.5 94.9 |
|
| ||||||
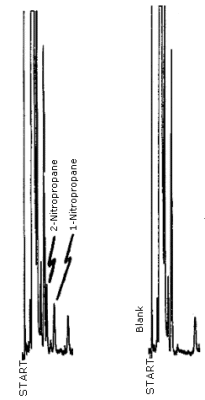
4.8. Chromatogram
A chromatogram of

5. References
- 5.1. Lee, D., 2-Nitropropane, Method 15, Organic Methods
Evaluation Branch, OSHA Analytical Laboratory, Salt Lake City, Utah,
Unpublished, September 1979.
5.2. "Occupational Health Guidelines for Chemical Hazards" NIOSH/OSHA, January 1981, DHHS (NIOSH) Publication No. 81-123.
5.3. Windholz, M., Ed. "Merck Index", 9th ed.; Merck and Co.: Rahway, NJ, 1979.