| Method no.: | 49 |
| Matrix: | Air |
| Target concentration: | 1 ppm (1.8 mg/m3) |
| Procedure: | Samples are collected by exposing 3M Ethylene Oxide Monitors #3551 for a measured period of time. Samples are desorbed with tetrahydrofuran. An aliquot of the desorbed sample is derivatized with heptafluorobutyric acid anhydride. The derivative is analyzed by gas chromatography using electron capture detection. |
| Sampling rate of monitor: (3M specification) |
49.3 mL/min at 760 mm Hg and 25°C |
| Minimum air velocity: (3M specification) |
15 ft/min for area samples |
| Reliable quantitation limit: (Section 1.2.3.) (based on an 8-h exposure at 760 mm Hg and 25°C) |
0.7 ppb (1.3 µg/m3) |
| Standard error of estimate at the target concentration: (Section 4.4.) |
6.4% |
| Special requirement: | It is recommended samples be refrigerated upon receipt by the laboratory until analyzed. |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: November 1984 | Chemist: Carl J. Elskamp |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
The 3M Ethylene Oxide Monitor was evaluated at a target concentration of 1 ppm. The monitor is a badge containing a chemically treated charcoal disk which converts adsorbed ethylene oxide to 2-bromoethanol (Ref. 5.1.). (It is presumed the charcoal is impregnated with hydrogen bromide, but the exact coating procedure is proprietary information.) Ethylene oxide is collected by diffusion, thus no sampling pumps are needed, but a minimum air velocity must exist during sampling to assure that badge starvation does not occur. The 2-bromoethanol formed on the sampler is less likely to migrate than ethylene oxide. The migration of ethylene oxide can be a major disadvantage in other methods that utilize untreated charcoal for collection (Refs. 5.2 and 5.3.) when samples have to be shipped to a laboratory for analysis. Also it is possible to sample for 8 h with one badge instead of using numerous samplers for the same period required for untreated charcoal tube methods.
The original analytical procedure for the monitors was developed
by 3M when the
An electron capture detector was considered for use in the analysis since it is much more sensitive than the flame ionization detector for 2-bromoethanol. The 3M method specifies the use of 10% (v/v) methylene chloride in methanol for the desorption solvent. Since methylene chloride is not compatible with an electron capture detector, other solvents were tested. Among those tried were acetone, isopropyl alcohol, tetrahydrofuran (THF), benzene, and mixtures of carbon disulfide in isopropyl alcohol. Several of these solvents appeared to desorb the 2-bromoethanol well, notably THF and acetone, but after repeated injections of desorbed samples, chromatography problems developed. These problems included drastic changes in detector response, loss of resolution, and loss of peak symmetry. These difficulties may have been due to unreacted hydrogen bromide which could also have been desorbed from the samplers. Attempts were made to destroy the excess hydrogen bromide by adding sodium carbonate, sodium bicarbonate, or ammonium acetate to the desorbed samples, but no improvement was made. At this point an attempt to form a derivative of 2-bromoethanol was considered.
The stable and volatile fluoroacyl derivative of 2-bromoethanol formed from heptafluorobutyric acid anhydride (HFAA) or heptafluorobutyrylimidazole (HFBI) which is analyzed in a concurrently evaluated ethylene oxide method utilizing a solid sorbent tube proved to be an excellent candidate for analysis by electron capture chromatography (Ref. 5.5.). Since the derivative is so heavily halogenated to give a high response, only a small aliquot of the desorbed sample is used, thus interferences are greatly diluted. Also the derivatized aliquot is washed with water to extract any water soluble material such as hydrogen bromide. Tetrahydrofuran gives consistently high desorption efficiencies. The only minor inconvenience is that a side reaction of THF with HBr occurs during the desorption process to form 4-bromobutanol. This is similarly derivatized as the 2-bromoethanol during the derivatization step and upon analysis elutes about 10 min after the 2-bromoethanol derivative. N,N-Dimethylformamide appears to be a good desorption solvent but chromatographic interferences are extracted from blank samplers which are significant at lower sample concentrations.
Thus, the 3M badges were successfully validated at a target concentration equivalent to 1 ppm (for an 8 h exposure) by desorbing the samplers with THF, derivatizing an aliquot of the sample with HFAA, and analyzing by gas chromatography with electron capture detection.
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
It has long been recognized that exposure to high levels of ethylene oxide can cause a variety of toxic effects including respiratory tract, eye, and skin irritations, nausea, vomiting, central nervous system depression and even death. Intermittent high exposures are also reported to cause neurological effects such as sensory motor neuropathies and seizures (Ref. 5.6.).
Recently major concern has focused on the mutagenic and carcinogenic properties exhibited by ethylene oxide. Quoting the June 22, 1984 publication of the new ethylene oxide standard from the Federal Register:
- The evidence suggests that EtO may cause cancer of the blood
(leukemia) as well as other organs in humans. In addition, EtO
exposure causes mutations, increases in the rate of chromosomal
aberration and
These conclusions are supported by a number of animal experiments
involving exposure to ethylene oxide by a variety of different
routes including: inhalation, subcutaneous injection, dermal
exposure and intragastric administration. Additional data is cited
in the publication of the standard to indicate that "virtually every
mutagenicity test system applied to EtO has shown the chemical to be
mutagenic." Several epidemiological studies indicate that excess
cancers may be occurring in the workplace due to ethylene oxide
exposure. Additional studies involving groups of workers exposed to
varying levels of ethylene oxide indicate an ethylene oxide
dose-dependent increase in
1.1.3. Potential workplace exposure
Ethylene oxide is a major industrial chemical with production volume ranking in the top 25 among all chemicals produced in the United States. Approximately 6.7 billion pounds were produced domestically by the most recent estimate (Ref. 5.4.). Over 99% of the total produced in the United States is used in the manufacture of other products. Approximately 70% of the total is used to produce ethylene glycol. Ethylene oxide is also used to produce non-ionic surface-active agents used in household detergents, ethanolamines, glycol ethers, di-, tri-, tetra-, and polyethylene glycols and crown ethers (Ref. 5.6.).
Although less than 1% of the total ethylene oxide produced in the United States is used as a sterilizing agent, this small sector represents the greatest number of potential work exposures. It is estimated that some 62,370 employees in 6237 hospitals in the United States are potentially exposed to ethylene oxide. Another 5000 workers are estimated to be potentially exposed to ethylene oxide in its use as a sterilizing agent in the medical products manufacturing industry (Ref. 5.4.).
A small number of workers in other industries are also potentially exposed to ethylene oxide during its use as a fumigant and a sterilizing agent. Spice manufacturing, libraries, museums, dairy packing and fur treatment are some of the industries and work settings in which ethylene oxide exposure can occur (Ref. 5.6.).
1.1.4. Physical properties (Ref. 5.7. unless otherwise noted)
| CAS no.: | 75-21-8 |
| molecular weight: | 44.05 |
| boiling point: | 10.4°C at 760 mm Hg |
| color: | colorless gas |
| density: | 0.8697 g/mL at 20°C |
| structural formula: | |
| vapor pressure: | 1094 mm Hg at 20°C |
| flash point (tag open cup): | <-18°C |
| odor (Ref. 5.3.): | ether-like |
| explosive limits air: | lower, 3% by volume upper, 100% by volume |
| synonyms (Ref. 5.4.): | dihydrooxirene, dimethylene oxide, EO, EtO,
ETO, oxane, oxiran, oxirane, oxidoethane, oxacyclopropane,
alpha/betaoxidoethane,
|
1.2. Limit Defining Parameters (The analyte air concentrations listed throughout this method are based on an exposure time of 8 h (at 760 mm Hg & 25°C), a desorption volume of 1.5 mL THF, and the derivatization of 25 µL of desorbed sample with 20 µL of HFAA contained in 1.0 mL of isooctane. The amounts are expressed as ethylene oxide, although the derivative is analyzed.)
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 0.2 pg per injection. This is the amount of analyte which will give a measurable response with the amounts of interferences present in a standard. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 0.03 µg per sample (0.7 ppb or 1.3 µg/m3). This is the amount of analyte spiked on the sampling device which allows recovery approximately equivalent to the detection limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 0.03 µg per sample (0.7 ppb or 1.3 µg/m3). This is the smallest amount of analyte which can be quantitated within the requirements of a recovery of at least 75% and a precision (±1.96 SD) of ±25% or better. (Section 4.3.)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Sensitivity
The sensitivity of the analytical procedure over the concentration range representing 0.5 to 2 times the target concentration based on the recommended exposure time is approximately 60,000 area units per µg/sample. This is determined by the slope of the calibration curve. (Section 4.3.) The sensitivity will vary with the particular instrument used in the analysis.
1.2.5. Recovery
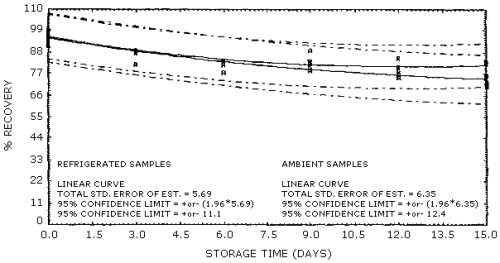
The recoveries of ethylene oxide from samples used in a 15-day storage test remained above 78% when the samples were stored at ambient temperatures (20-26°C) in a closed drawer. (Section 4.4.) The recovery of analyte from the collection medium during storage must be 75% or greater.
1.2.6. Precision
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1, and 2 times the target concentration is 0.009. (Section 4.3.)
1.2.7. Precision (overall procedure)
The precision at the 95% confidence level for the 15-day storage test is ±12.4%. (Section 4.4.) This includes an additional ±5% for sampling error. The overall procedure must provide results that are ±25% or better at the 95% confidence level.
1.2.8. Reproducibility
Six samples collected from a controlled test atmosphere and a draft copy of this procedure were given to a chemist unassociated with this evaluation. The samples were analyzed after 15 days of storage at 0°C. The average recovery was 99.0% with a standard deviation of 3.1%. (Section 4.5.)
1.3. Advantages
- 1.3.1. The badge provides a convenient method for sampling since
no sampling pumps are required.
1.3.2. Sampler exposure times can be longer for the badge than the recommended maximum sampling times for untreated charcoal tubes, thus fewer samples are required.
1.4. Disadvantages
- 1.4.1. A minimum face velocity is required for reliable
sampling. This may not be obtainable in all field situations.
1.4.2. The analysis involves the formation of a derivative of 2-bromoethanol which is more time-consuming than direct analysis.
2. Sampling Procedure
- 2.1. Apparatus
Samples are collected on 3M Ethylene Oxide Monitors #3551.
2.2. Reagents
None required
2.3. Technique
- 2.3.1. The monitor and closure cap are removed from the
resealable bag. The cap remains with the bag.
2.3.2. The monitor is removed from its sealed package.
2.3.3. The exposure start time is recorded on the back of the monitor.
2.3.4. The monitor is attached to the worker near the breathing zone. The white film (wind screen) and ring must not be removed until the sampling period is terminated.
2.3.5. After sampling, the monitor is removed from the worker. The white film and its retaining ring should be immediately removed from the monitor with a coin or other suitable device.
2.3.6. The closure cap is snapped onto the monitor and the ports firmly closed.
2.3.7. The time at the end of the sampling period is recorded on the back of the monitor.
2.3.8. The sampling information is entered on the original bag. This information should include atmospheric station pressure or elevation of the sampling site. The capped monitor is placed in the bag and the bag is sealed with OSHA Form 21. The white films and retaining rings are discarded.
2.3.9. At least one blank monitor is submitted with each sample set.
2.4. Capacity
The sampling capacity of the monitor is 3200 µg of ethylene oxide as reported by 3M. This is equivalent to sampling a 75-ppm atmosphere for 8 h.
2.5. Desorption efficiency
- 2.5.1. The average desorption efficiency of ethylene oxide from
spiked monitors is 96.9% over the range of 0.5 to 2 times the target
concentration. (Section 4.6.)
2.5.2. The time required for desorption must be determined for each lot of monitors. It was found that for newer lots, the desorption took about 24 h before the efficiency leveled off.
2.6. Sampling rate and minimum air velocity required (from 3M)
- 2.6.1. The sampling rate at 760 mm Hg and 25°C is 49.3 mL/min.
2.6.2. The minimum air velocity required for area samples is 15 ft/min.
2.7. Interferences (sampling)
- 2.7.1. It is not known if any compound(s) will severely
interfere with the collection of ethylene oxide.
2.7.2. Suspected interferences should be reported to the laboratory with submitted samples.
2.8. Safety precautions
- 2.8.1. Attach the sampling equipment to the employee so that it
will not interfere with work performance or safety.
2.8.2. Follow all safety procedures that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus: The following are required for analysis:
- 3.1.1. A GC equipped with an electron capture detector. For this
evaluation, a
3.1.2. A GC column capable of separating the HFAA derivative of 2-bromoethanol from isooctane and any interferences. A 10-ft × 1/8-in. stainless steel column packed with 10% SP-1000 coated on 80/100 Supelcoport was used in this evaluation.
3.1.3. An electronic integrator or some other suitable method of measuring peak areas or heights.
3.1.4. Small vials with Teflon-lined caps capable of holding 4 mL. WISP vials were used in this evaluation.
3.1.5. Small vials with Teflon-lined caps capable of holding 2
mL.
3.1.6. A dispenser capable of delivering 1.5 mL THF into the monitors for desorption and into vials for preparation of standards. A 2.5-mL Gas-Tight Hamilton syringe was used in this evaluation.
3.1.7. Dispensers, one capable of delivering 20 µL and another capable of delivering 25.0 µL. SMI digital adjust Micro/ Pettors were used in this evaluation.
3.1.8. Syringes for preparation of standards and for injection of samples and standards into a GC. A 10-µL syringe was used for standard preparation and a 1-µL syringe was used for the injections in this evaluation.
3.1.9. Volumetric flasks and pipets to dilute the 2-bromoethanol.
3.1.10. Disposable dropping pipets.
3.2. Reagents
- 3.2.1. Tetrahydrofuran, isooctane and water; reagent grade.
3.2.2. 2-Bromoethanol, reagent grade. Kodak lot A10B was used.
3.2.3. Heptafluorobutyric acid anhydride (HFAA). HFAA from Pierce Chemical Company was used.
3.2.4. Magnesium sulfate, reagent grade.
3.3. Standard preparation
- 3.3.1. Stock standards are prepared by diluting 1.0 mL of
2-bromoethanol to 100 mL with THF.
3.3.2. Analytical standards are prepared by injecting microliter amounts of stock standards into WISP vials containing 1.5 mL of THF. A 25-µL aliquot of this solution is then added to another WISP vial containing 1.0 mL of isooctane. To this vial, 20 µL of HFAA is added and the vial is capped and shaken for a few seconds. After 10 min, 1 mL of water is added and the vial is shaken for 10 s. The isooctane layer is transferred with a disposable pipet to an autosampler vial containing approximately 50 mg of magnesium sulfate. The vial is capped and shaken for a few seconds. This solution is injected into a GC.
3.3.3. Analytical standard concentrations should bracket sample concentrations. Thus, if samples fall out of the range of prepared standards, additional standards must be prepared to ascertain detector response.
3.4. Sample preparation
- 3.4.1. Add 1.5 mL of THF through one of the ports in the monitor
using the same dispenser as used for preparing standards.
3.4.2. The port is immediately plugged. The monitors are desorbed for a period of time as determined in Section 2.5.
3.4.3. The desorption solution is transferred to a WISP vial and a 25-µL aliquot is derivatized as in 3.3.2.
3.5. Analysis
- 3.5.1. GC conditions
| zone temperatures (°C): | 100 (column) 200 (injector) 300 (detector) |
| gas flow (mL/min): | 25 (argon/methane(95/5)) |
| injection volume: | 0.4 µL |
| column: | 10-ft × 1/8-in. SS, 10% SP-1000 on 80/100 Supelcoport |
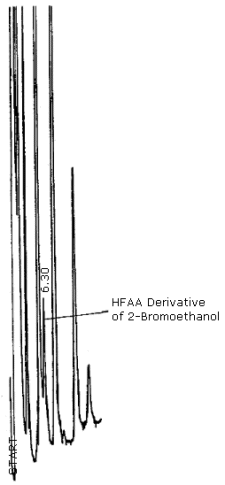
| retention times (min): | 6.2 (2-bromoethanol deriv.) 16.5 (4-bromobutanol deriv.) |
| chromatogram: | Section 4.7. |
3.5.2. Peak areas (or heights) are measured by an integrator or other suitable means.
3.5.3. A calibration curve is constructed by plotting peak areas (or heights) of standard injections versus µg ethylene oxide per sample. Sample concentrations must be bracketed by standards.
3.6. Interferences (analytical)
- 3.6.1. Any compound that responds on an electron capture
detector and has the same general retention time as the HFAA
derivative of 2-bromoethanol is a potential interference. Possible
interferences should be reported to the laboratory with submitted
samples by the industrial hygienist. These interferences should be
considered before samples are desorbed.
3.6.2. GC parameters (i.e. column and column temperature) may be changed to possibly circumvent interferences.
3.6.3. Retention time on a single column is not considered proof of chemical identity. Samples should be confirmed by GC/MS if possible.
3.7. Calculations
The analyte concentration for samples is obtained from the appropriate calibration curve in terms of micrograms per sample, uncorrected for desorption efficiency. The air concentrations are calculated using the following formulae. The amount of analyte found on the samples is corrected by subtracting the amount (if any) found on the blank.
| mg/m3 = | (micrograms per sample)
(liters of air sampled) (desorption efficiency) |
Liters of air sampled is found by the following:
| liters sampled | = | (T)(0.0493)((K/298)^1.5)(760/P) | |
| where T | = | Exposure time (minutes) | |
| K | = | Sampling site temp (EK) | |
| P | = | Sampling site pressure (mm Hg) | |
| 0.0493 | = | sampling rate in L/min at 760 mm Hg and 25°C | |
ppm = (mg/m3)(24.46/44.01) = (mg/m3)(0.5553)
3.8. Safety precautions (analytical)
- 3.8.1. Avoid skin contact and inhalation of all chemicals.
3.8.2. Restrict the use of all chemicals to a fume hood if possible.
3.8.3. Wear safety glasses and a lab coat at all times while in the lab area.
4. Backup Data
- 4.1. Detection limit data
The injection size listed in the analytical procedure (0.4 µL) was
used in the determination of the detection limit of the analytical
procedure. The detection limit of 0.2 pg of ethylene oxide was
determined by analyzing a dilute standard equivalent to 0.03 µg of
ethylene oxide per sample. Shown in Figure 4.1. is a chromatogram of
this analysis made on a
4.2. Detection limit of the overall procedure and reliable quantitation limit data
Six samples were prepared by injecting 0.03 µg of ethylene oxide into six monitors. The samples were then later desorbed and analyzed to determine the amount recovered. Since recovery was high and approximately equal to the detection limit of the analytical procedure, the detection limit of the overall procedure and the reliable quantitation limit are taken to be 0.03 µg per sample (0.7 ppb or 1.3 µg/m3). The results of this study are given in Table 4.2.
Detection Limit Data
|
| |||
| % recovery | statistics | ||
|
| |||
| 96.3 98.7 91.7 93.0 96.0 91.7 |
SD 1.96 SD |
= = = |
94.6 2.9 5.7 |
|
| |||
4.3. Sensitivity and Precision (analytical method only)
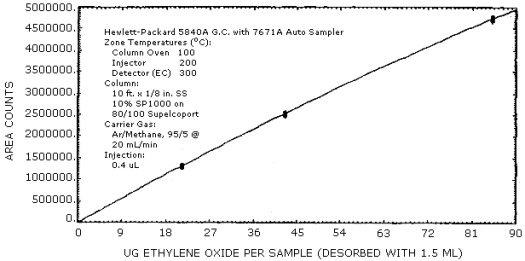
The sensitivity and precision of the analytical procedure were determined from multiple injections of analytical standards. These data are given in Table 4.3. and Figure 4.3.
Sensitivity and Precision Data
|
| |||
| × target conc. µg/sample ppm |
0.5× 21.3 0.50 |
1× 42.6 1.00 |
2× 85.2 2.00 |
|
| |||
| area counts SD CV |
1311000 1289000 1313000 1339000 1314000 1324000 1315000 16500 0.0125 |
2506000 2528000 2538000 2546000 2497000 2555000 2528000 22800 0.009 |
4714000 4716000 4765000 4727000 4730000 4716000 4728000 19300 0.004 |
|
| |||
4.4. Recovery data (storage)
Storage samples were generated from test atmospheres (air at approximately 80% relative humidity) containing ethylene oxide at approximately 8 ppm. The samples were generated at ambient temperatures (20-25°C) and pressures (660-665 mm Hg) by exposing the badges for 1 h. The amount of ethylene oxide thus collected was equivalent to exposing the badges to a 1 ppm atmosphere for 8 h. Six samples were analyzed immediately after generation, 15 were stored in a refrigerator at 2°C, and 15 were stored in a closed drawer at ambient temperature. The results of recovery versus storage time under both conditions are given in Table 4.4. and shown graphically in Figure 4.4.
Storage Tests
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 0 3 6 9 12 15 |
99.0 93.9 89.1 83.4 83.4 85.1 82.7 |
99.6 96.5 88.2 83.6 83.7 80.0 74.8 |
91.5 96.6 87.9 83.1 81.1 80.5 83.9 |
99.0 93.9 81.8 81.7 89.2 75.0 76.3 |
99.6 96.5 88.7 82.0 82.4 76.1 72.2 |
91.5 96.6 88.2 77.6 78.8 78.5 70.8 | |
|
| |||||||
4.5. Reproducibility
Six samples were collected by exposing the monitors for 1 h to a controlled test atmosphere (80% R.H., 23.3°C, 651 mm Hg) containing 7.4 ppm ethylene oxide. The samples were analyzed by a chemist unassociated with this evaluation. The results are given in Table 4.5.
Reproducibility
|
| ||||||
sample no. |
found µg/L |
expected µg/L |
% recovery | |||
|
| ||||||
| 1 2 3 4 5 6 |
13.82 13.10 13.49 13.25 13.02 13.00 |
13.35 13.35 13.35 13.35 13.35 13.35 | 103.5 98.1 101.0 99.3 97.5 94.4 | |||
| ||||||
|
| ||||||
4.6. Desorption efficiency
The desorption efficiency was determined by injecting known amounts of ethylene oxide standards (in THF) into the monitors. The samples were analyzed the next day after storing at room temperature in a closed drawer.
Desorption Efficiency Data
|
| |||
| × target conc. µg/sample ppm |
0.5× 21.3 0.50 |
1× 42.6 1.00 |
2× 85.2 2.00 |
|
| |||
| desorption efficiency, % SD |
96.6 99.0 98.6 97.0 95.7 94.9 97.0 1.6 |
95.1 95.5 95.5 97.3 95.1 95.1 95.6 0.86 |
97.4 99.5 97.7 98.0 98.6 98.2 98.2 0.74 |
|
| |||
4.7. Chromatogram
A chromatogram is shown in Figure 4.7. The chromatogram represents a 0.4-µL injection of a standard equivalent to 42.6 µg of ethylene oxide per sample. This concentration is equal to 1.0 ppm for an 8-h exposure of a monitor.

5. References
- 5.1. 3M/Occupational Health and Safety Products Division
"Diffusional Monitoring For Ethylene Oxide", 3M Center, St. Paul, MN
55144
5.2. Qazi, A.H,; Ketcham, N.H. Am. Ind. Hyg. Assoc. J. (1977), (38), 635-647.
5.3. Potter, Wayne "OSHA Method No. 30, Ethylene Oxide", August 1981, OSHA Analytical Laboratory, Salt Lake City, Utah 84165.
5.4. "Occupational Exposure to Ethylene Oxide", "Federal Register, June 22, 1984, (49), 25734-809.
5.5. Cummins, K.J. "OSHA Method No. 50, Ethylene Oxide", September 1984, OSHA Analytical Laboratory, Salt Lake City, Utah 84165.
5.6. "Current Intelligence Bulletin No. 25, Ethylene Oxide", May 22, 1981, U.S. Dept. of Health and Human Services, Public Health Service, Center for Disease and Control, NIOSH.
5.7. J.N. Cawse in "Kirk-Othmer Encyclopedia of Chemical Technology", Vol. 9, pp. 432-471, 3rd ed., John Wiley and Sons, N.Y. 1980.