| Method no.: | 56 |
| Matrix: | Air |
| Target concentration: | 1 ppm (2.21 mg/m3) |
| Procedure: | Air samples are collected by drawing known volumes of air through sampling tubes containing charcoal adsorbent which has been coated with 4-tert-butylcatechol. The samples are desorbed with carbon disulfide and then analyzed by gas chromatography using a flame ionization detector. |
| Recommended air volume and sampling rate: |
3 L at 0.05 L/min |
| Detection limit of the overall procedure: |
90 ppb (200 µg/m3) |
| Reliable quantitation limit: | 155 ppb (343 µg/m3) |
| Standard error of estimate at the target concentration: (Section 4.6.1.) |
6.5% |
| Special requirements: | The sampling tubes must be obtained from the Salt Lake City Analytical Laboratory. Collected samples should be stored in a freezer. |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: December 1985 | Chemist: Warren Hendricks |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
This work was undertaken to develop a sampling and analytical
procedure for
The current method recommended by OSHA for collecting
The stability of collected samples has been significantly
improved through the use of a specially cleaned charcoal which is
coated with 4-tert-butylcatechol (TBC). TBC is a polymerization
inhibitor for
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy).
Symptoms of human exposure to
NIOSH recommends that
1.1.3. Potential workplace exposure
In 1984, 2.53 billion pounds of rubber grade butadiene were
produced. This amount was only 3.7% less than the average yearly
amount produced during the past decade of
About 90% of the annual production of
A NIOSH survey, that was conducted from 1972 to 1974, estimated
that approximately 65,000 workers were potentially exposed to
1.1.4. Physical properties (Ref. 5.1.)
| CAS no.: | 106-99-0 |
| molecular weight: | 54.09 |
| appearance: | colorless gas |
| boiling point: | -4.41°C (760 mm Hg) |
| freezing point: | -108.9°C |
| vapor pressure: | 2 atm at 15.3°C 5 atm at 47.0°C |
| explosive limits: | 2 to 11.5% (by volume in air) |
| odor threshold: | 1.3 ppm |
| structural formula: | H2C:CHCH:CH2 |
| synonyms: | biethylene; bivinyl; butadiene; divinyl;
|
1.2. Limit defining parameters (The analyte air concentrations listed throughout this method are based on an air volume of 3 L and a desorption volume of 1 mL. Air concentrations listed in ppm are referenced to 25°C and 760 mm Hg.)
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure was 304 pg per
injection. This was the amount of
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure was 0.60 µg per sample (90 ppb or 200 µg/m3). This amount was determined graphically. It was the amount of analyte which, when spiked on the sampling device, would allow recovery approximately equivalent to the detection limit of the analytical procedure. (Section 4.1.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit was 1.03 µg per sample (155 ppb or 343 µg/m3). This was the smallest amount of analyte which could be quantitated within the limits of a recovery of at least 75% and a precision (±1.96 SD) of ±25% or better. (Section 4.2.)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Sensitivity
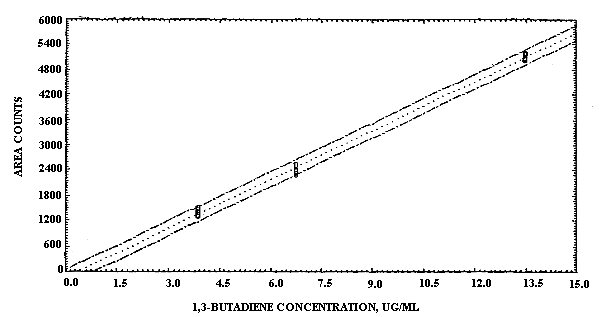
The sensitivity of the analytical procedure over a concentration range representing 0.6 to 2 times the target concentration, based on the recommended air volume, was 387 area units per µg/mL. This value was determined from the slope of the calibration curve. (Section 4.3.) The sensitivity may vary with the particular instrument used in the analysis.
1.2.5. Recovery
The recovery of
1.2.6. Precision (analytical method only)
The pooled coefficient of variation obtained from replicate determinations of analytical standards over the range of 0.6 to 2 times the target concentration was 0.011. (Section 4.3.)
1.2.7. Precision (overall procedure)
The precision at the 95% confidence level for the refrigerated temperature storage test was ±12.7%. (Section 4.6.1.) This value includes an additional ±5% for sampling error. The overall procedure must provide results at the target concentrations that are ±25% at the 95% confidence level.
1.2.8. Reproducibility
Samples collected from a controlled test atmosphere and a draft copy of this procedure were given to a chemist unassociated with this evaluation. The average recovery was 97.2% and the standard deviation was 6.2%. (Section 4.7.)
1.3. Advantages
- 1.3.1. The sampling and analytical procedure permits
determination of
1.3.2. Samples are relatively stable following storage at ambient temperature for 17 days.
1.4. Disadvantage
The recommended sampling tubes must be obtained from the Salt Lake City Analytical Laboratory.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. Samples are collected by use of a personal sampling pump
that can be calibrated to within ±5% of the recommended 0.05 L/min
sampling rate with the sampling tube in line.
2.1.2. Samples are collected with laboratory prepared sampling
tubes. The sampling tube is constructed of silane-treated glass and
is about 5-cm long. The i.d. is 4 mm and the o.d. is 6 mm. One end
of the tube is tapered so that a glass wool end plug will hold the
contents of the tube in place during sampling. The opening in the
tapered end of the sampling tube is at least one-half the i.d. of
the tube (2 mm). The other end of the sampling tube is open to its
full 4-mm i.d. to facilitate packing of the tube. Both ends of the
tube are fire-polished for safety. The tube is packed with 2
sections of pretreated charcoal which has been coated with TBC. The
tube is packed with a 50-mg backup section, located nearest the
tapered end, and with a
2.2. Reagents
None required
2.3. Technique
- 2.3.1. Properly label the sampling tube before sampling and then
remove the plastic end caps.
2.3.2. Attach the sampling tube to the pump using a section of flexible plastic tubing such that the large front section of the sampling tube is exposed directly to the atmosphere. Do not place any tubing ahead of the sampling tube. The sampling tube should be attached in the worker's breathing zone in a vertical manner such that it does not impede work performance.
2.3.3. After sampling for the appropriate time, remove the sampling tube from the pump and then seal the tube with plastic end caps. Wrap the tube lengthwise with an official OSHA seal (Form 21).
2.3.4. Include at least one blank for each sampling set. The blank should be handled in the same manner as the samples with the exception that air is not drawn through it.
2.3.5. List any potential interferences on the sample data sheet.
2.3.6. The samples require no special shipping precautions under normal conditions. The samples should be refrigerated if they are to be exposed to higher than normal ambient temperatures. If the samples are to be stored before they are shipped to the laboratory, they should be kept in a freezer. The samples should be placed in a freezer upon receipt at the laboratory.
2.4. Breakthrough (Breakthrough was defined as the relative amount of analyte found on the backup section of the tube in relation to the total amount of analyte collected on the sampling tube.)
Five-percent breakthrough occurred after sampling a test atmosphere
containing 2.0 ppm
Breakthrough studies have shown that the recommended sampling
procedure can be used at air concentrations higher than the target
concentration. The sampling time, however, should be reduced to 45 min
if both the expected
2.5. Desorption efficiency
The average desorption efficiency for
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 3 L.
2.6.2. The recommended sampling rate is 0.05 L/min for 1 h.
2.7. Interferences (sampling)
There are no known interferences to the sampling method.
2.8. Safety precautions (sampling)
- 2.8.1. Attach the sampling equipment to the worker in such a
manner that it will not interfere with work performance or safety.
2.8.2. Follow all safety practices that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A gas chromatograph (GC), equipped with a flame
ionization detector (FID). A Hewlett-Packard Model 5840A GC was used
for this evaluation. Injections were performed using a
Hewlett-Packard Model 7671A automatic sampler.
3.1.2. A GC column capable of resolving the analytes from
potential interferences. A
3.1.3. Two milliliter glass vials with Teflon-lined caps.
3.1.4. Disposable Pasteur-type pipets, volumetric flasks, pipets and syringes for preparing samples and standards, making dilutions and performing injections.
3.2. Reagents
- 3.2.1. Carbon disulfide. Fisher Scientific Company A.C.S.
Reagent Grade solvent was used in this evaluation. The benzene
contaminant that was present in the carbon disulfide was used as an
internal standard (ISTD) in this evaluation.
3.2.2. Nitrogen, hydrogen, and air, GC grade.
3.2.3. 1,3-Butadiene of known high purity. Matheson Gas Products,
CP Grade
3.3. Standard preparation
- 3.3.1. Prepare standards by diluting known volumes of
1,3-buta-diene gas with carbon disulfide. This can be accomplished
by injecting the appropriate volume of
3.3.2. The mass of
| where | 22.41 MV BP T |
= = = = |
molar volume at STP ambient molar volume ambient barometric pressure ambient temperature |
µg/µL = 54.09/MV
µg per standard = (µg/µL)(µL)(p)
| where | µg/µL µL p |
= = = |
ambient density of
µL of purity of |
3.4. Sample preparation
- 3.4.1. Transfer the c section of the sampling tube to a 2-mL
vial. Place the 50-mg section in a separate vial. If the glass wool
plugs contain a significant amount of charcoal, place them with the
appropriate sampling tube section.
3.4.2. Add 1 mL of carbon disulfide to each vial.
3.4.3. Seal the vials with Teflon-lined caps and then allow them to desorb for 1 h. Shake the vials by hand with vigorous force several times during the desorption time.
3.4.4. If it is not possible to analyze the samples within 4 h of desorption, separate the carbon disulfide from the charcoal, using a disposable Pasteur-type pipet, following the 1-h desorption time. This separation will improve the stability of desorbed samples. (Tables 4.5.1.2. and 4.5.1.3.)
3.4.5. Save the used sampling tubes to be cleaned and repacked with fresh adsorbent.
3.5. Analysis
- 3.5.1. GC Conditions
| column temperature: | 95°C |
| injector temperature: | 180°C |
| detector temperature: | 275°C |
| carrier gas flow rate: | 30 mL/min |
| injection volume: | 0.80 µL |
| GC column: | 20-ft × 1/8-in. o.d. stainless steel GC column
containing 20%FFAP on 80/100 Chromosorb |
3.5.2. Chromatogram See Section 4.9.
3.5.3. Use a suitable method, such as electronic integration or peak heights, to measure detector response.
3.5.4. Prepare a calibration curve using several standard solutions of different concentrations. Prepare the calibration curve daily. Program the integrator to report the results in µg/mL.
3.5.5. Bracket sample concentrations with standards.
3.6. Interferences (analytical)
- 3.6.1. Any compound with the same general retention time as the
analyte and which also gives a detector response is a potential
interference. Possible interferences should be reported to the
laboratory with submitted samples by the industrial hygienist.
3.6.2. GC parameters (temperature, column, etc.) may be changed to circumvent interferences.
3.6.3. A useful means of structure designation is GC/MS. It is recommended that this procedure be used to confirm samples whenever possible.
3.7. Calculations
- 3.7.1. Results are obtained by use of calibration curves.
Calibration curves are prepared by plotting detector response
against concentration for each standard. The best line through the
data points is determined by curve fitting.
3.7.2. The concentration, in µg/mL, for a particular sample is determined by comparing its detector response to the calibration curve. If any analyte is found on the backup section, this amount is added to the amount found on the front section. Blank corrections should be performed before adding the results together.
3.7.3. The
| where | A = µg/mL from Section 3.7.2. B = desorption volume C = liters of air sampled D = desorption efficiency |
3.7.4. The following equation can be used to convert results in mg/m3 to ppm:
| where | mg/m3 | = | result from Section 3.7.3. |
| 24.46 | = | molar volume of an ideal gas at 760 mm Hg and 25°C |
3.8. Safety precautions (analytical)
- 3.8.1. Avoid skin contact and inhalation of all chemicals.
3.8.2. Restrict the use of all chemicals to a fume hood whenever possible.
3.8.3. Wear safety glasses and a lab coat in all lab areas.
4. Backup Data
- 4.1. Detection limit data
- 4.1.1. Detection limit of the analytical procedure
The injection size recommended in the analytical procedure (0.80
µL) was used in the determination of the detection limit for the
analytical procedure. The detection limit of the analytical
procedure was 304 pg per injection. This was the amount of
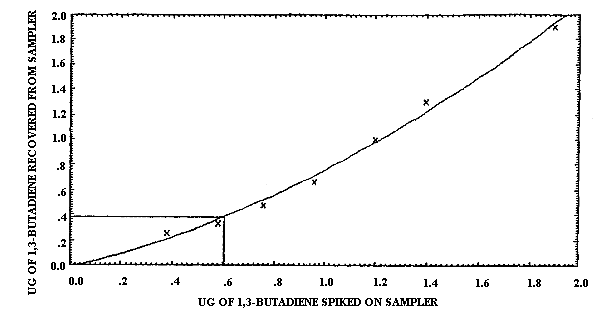
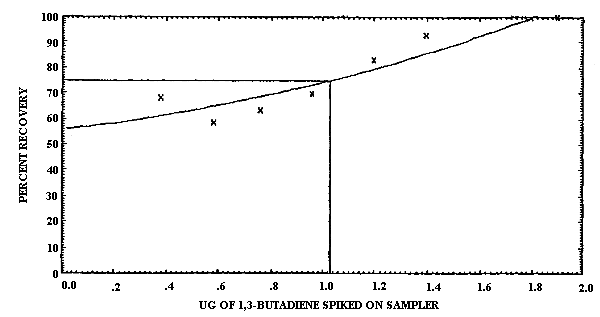
4.1.2. Detection limit of the overall procedure
The injection size recommended in the analytical procedure (0.80
µL) was used in the determination of the detection limit of the
overall procedure. 1,3-Butadiene was diluted for use in this study
by adding the pure analyte to a sealed, silanized vial containing
air and a few crystals of TBC. Samples were prepared by injecting
Detection Limit Data
|
| |||
| sample number |
µg spiked |
µg recovered |
percent recovered |
|
| |||
| 1 2 3 4 5 6 7 |
0.38 0.58 0.76 0.96 1.2 1.4 1.9 |
0.26 0.34 0.48 0.67 1.0 1.3 1.9 |
68.4 58.6 63.2 69.8 83.3 92.8 100.0 |
|
| |||
The detection limit of the overall procedure was determined graphically (Figure 4.1.2.) from the data in Table 4.1.2. This amount was 0.60 µg per sample.
4.2. Reliable quantitation limit data
The recommended injection size of 0.80 µL was used in the
determination of the reliable quantitation limit (RQL). The amount of
Reliable Quantitation Limit Data
|
| ||||||
| sample number |
µg spiked |
µg recovered |
percent recovered | |||
|
| ||||||
| 1 2 3 4 5 6 |
1.03 1.03 1.03 1.03 1.03 1.03 |
0.854 0.754 0.829 0.779 0.836 0.836 |
82.9 73.2 80.5 75.6 81.2 81.2 | |||
| 1.03 | 0.815 | 79.1 | ||||
| ||||||
|
| ||||||
4.3. Sensitivity and precision (analytical method only)
The sensitivity and precision of the analytical procedure were evaluated by performing multiple injections of analytical standards at 0.6, 1, and 2 times the target concentration. The standards were prepared by injecting appropriate amounts of 1,3-buta-diene gas diluted with carbon disulfide. The data are presented in Table 4.3. and also in Figure 4.3. The ISTD data are the results of an internal standard calibration using the benzene contaminant present in carbon disulfide as the internal standard.
1,3-Butadiene Sensitivity and Precision Data
|
| ||||||
| 0.6× | 1× | 2× | ||||
| 3.86 µg/sample | 6.75 µg/sample | 13.5 µg/sample | ||||
| ISTD | area | ISTD | area | ISTD | area | |
|
| ||||||
CV |
3.85 3.89 3.85 3.81 3.81 3.95 3.86 0.0138 0.011 |
1332 1509 1507 1345 1416 1354 |
6.66 6.73 6.78 6.78 6.86 6.75 6.76 0.00977 |
2371 2386 2369 2393 2529 2327 |
13.4 13.5 13.7 13.5 13.6 13.3 13.5 0.0105 |
5190 5167 5076 5097 5045 5087 |
|
| ||||||
The sensitivity for
4.4. Breakthrough data
Breakthrough was defined as the relative amount of
Three breakthrough studies were performed at twice the target level
with the recommended air sampler. The test atmospheres were generated
by diluting the effluent of a gas cylinder containing 100 ppm of
1,3-Butadiene Breakthrough at Twice the Target Concentration
|
| |||
| sampling time, min |
amt. on
|
amt. on 50-mg section, µg | breakthrough, % |
|
| |||
| 91 124 155 60 91 121 50 76 92 105 125 |
23.4 27.8 30.4 14.6 21.6 25.5 8.6 13.0 14.4 15.7 17.0 |
0.0 3.1 6.1 0.0 1.2 3.5 0.0 0.0 0.6 2.2 2.4 |
0.0 10.0 16.7 0.0 5.3 12.1 0.0 0.0 4.0 12.3 12.4 |
|
| |||
When the results of the three studies were combined, 5%
breakthrough occurred after sampling for 90 min. The air volume
sampled after this time was 4.5 L and the amount of
Additional breakthrough studies were performed at concentrations higher than twice the target level in order to determine if the recommended sampling procedure would be reliable at those concentrations. The test atmospheres used in these studies were generated and their concentrations were determined using the techniques previously described. Percent recovery values were calculated using sample results and the actual concentration of the test atmospheres. The sampling rates were about 0.05 L/min. The results of these studies are presented in Tables 4.4.2. through 4.4.4.
1,3-Butadiene Breakthrough Study at 7.3 ppm
(Relative Humidity = 77% at 22°C)
|
| |||
| sampling time, min |
air volume sampled, L |
percent breakthrough |
percent recovery |
|
| |||
| 15 30 45 60 75 90 |
0.73 1.6 2.2 3.1 3.5 4.5 |
0.0 0.0 0.0 0.0 0.6 8.4 |
80.2 94.2 96.8 99.4 96.7 95.8 |
|
| |||
Five-percent breakthrough occurred after sampling for 84 min. At
the end of this time, 4.2 L of air had been sampled and 68 µg of
1,3-Butadiene Breakthrough Study at 32 ppm
(Relative Humidity = 47% at 24°C)
|
| |||
| sampling time, min |
air volume sampled, L |
percent breakthrough |
percent recovery |
|
| |||
| 15 46 60 90 105 120 155 |
0.71 2.3 3.2 4.3 5.2 6.3 8.2 |
0.0 0.0 0.0 0.0 0.0 0.0 0.0 |
87.3 87.2 91.2 94.8 95.0 97.5 93.0 |
|
| |||
No breakthrough was observed, even after sampling for 155 min. This
data shows that, at low relative humidity, the recommended sampling
media has considerable capacity for
1,3-Butadiene Breakthrough Study at 36 ppm
(Relative Humidity = 90% at 21°C)
|
| |||
| sampling time, min |
air volume sampled, L |
percent breakthrough |
percent recovery |
|
| |||
| 36 47 60 75 90 105 121 |
1.9 2.2 3.0 3.9 4.3 5.8 6.3 |
0.0 0.0 21.6 30.0 30.6 32.1 31.1 |
105.8 98.8 90.2 96.0 76.4 57.6 56.7 |
|
| |||
It is apparent from the data in Tables 4.4.2. through 4.4.4. that
the recommended sampling and analytical method can be used at
4.5. Desorption efficiency and stability of desorbed samples
- 4.5.1. Pretreated charcoal coated with TBC
The desorption efficiency of
The stability of desorbed samples was investigated by reanalyzing
the target concentration desorption samples at various times after
carbon disulfide addition. Freshly prepared standards were used for
each analysis. The sample vials were resealed immediately after each
analysis. The results of this study are presented in Table 4.5.1.2.
The percent recovery is based on the theoretical amount of
The Desorption Efficiency
of 1,3-Butadiene from Charcoal Coated with TBC
|
| |||
| × target conc. µg/sample |
0.6× 3.86 |
1× 6.75 |
2× 13.5 |
|
| |||
| desorption efficiency, % |
94.3 95.4 96.4 96.9 94.8 96.9 95.8 95.8 |
100.0 97.0 102.0 96.0 94.3 98.8 98.0 98.0 |
97.5 97.5 95.8 95.2 93.4 92.5 95.3 95.3 |
|
| |||
The Stability (% Recovery) of 1,3-Butadiene
After Desorption from Charcoal Coated with TBC
|
| |||||||
| time | sample number | ||||||
| h | 1 | 2 | 3 | 4 | 5 | 6 | |
|
| |||||||
| 1 4 9 16 24 58 |
100.0 98.7 90.2 84.2 82.4 66.8 |
97.0 95.1 89.4 92.2 76.8 50.4 |
102.0 97.7 92.2 84.1 79.7 52.3 |
96.0 89.0 88.7 81.3 76.6 61.5 |
94.3 88.6 88.0 80.3 72.3 60.6 |
98.8 87.4 89.4 86.6 79.0 64.2 |
98.0 92.8 89.6 83.1 77.8 59.3 |
|
| |||||||
To determine if the stability of desorbed samples could be
improved, the following experiment was performed: twelve samples
were prepared by injecting
Effect of Charcoal on the Stability of Butadiene in CS2
|
| |||||||||
| storage time h |
CS2/charcoal separated |
CS2/charcoal not separated | |||||||
| 1 | 2 | 3 | 1 | 2 | 3 | ||||
|
| |||||||||
| 6 28 |
93.1 88.9 |
91.8 90.1 |
93.5 92.4 |
92.8 90.5 |
93.6 76.8 |
91.3 74.0 |
93.1 76.2 |
92.7 75.7 | |
|
| |||||||||
It appears that the stability of desorbed samples can be improved by separating the carbon disulfide from the charcoal.
4.5.2. Untreated charcoal
The desorption efficiency of
The stability of
The Desorption Efficiency
of 1,3-Butadiene from SKC, Inc. Lot 120 Charcoal
|
| |||
| × target conc. µg/sample |
0.6× 3.86 |
1× 6.75 |
2× 13.5 |
|
| |||
| desorption efficiency, % |
61.6 66.7 61.5 54.4 52.3 51.7 58.0 |
67.3 64.1 61.7 62.0 57.9 53.3 61.0 |
67.1 65.0 62.8 61.4 58.7 58.8 62.3 |
|
| |||
The Stability of 1,3-Butadiene After Desorption
From SKC, Inc. Lot 120 Charcoal (percent recovery)
|
| |||||||
| storage time | sample number | ||||||
| h | 1 | 2 | 3 | 4 | 5 | 6 | |
|
| |||||||
| 5 | 43.8 | 44.0 | 40.1 | 40.6 | 38.8 | 38.4 | 41.0 |
|
| |||||||
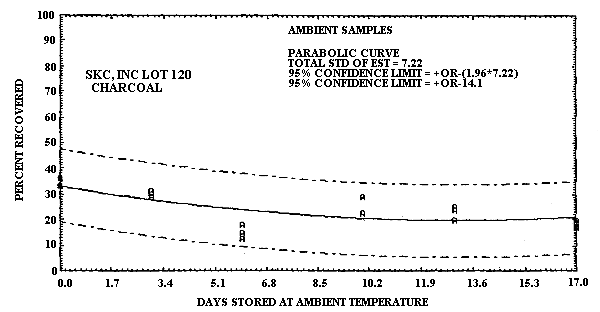
These data show that SKC, Inc. Lot 120 charcoal is inadequate for this application because of sample instability.
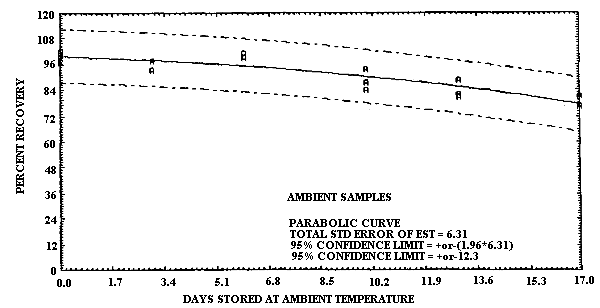
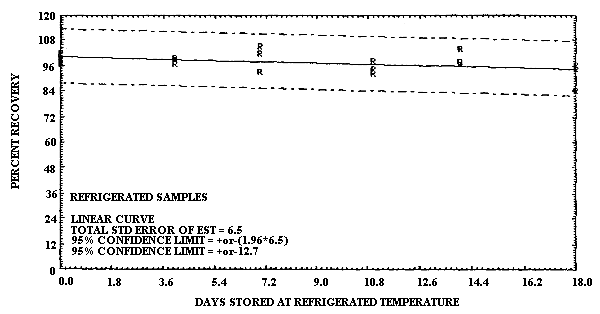
4.6. Storage data
- 4.6.1. Pretreated charcoal coated with TBC
The test atmosphere was generated by diluting the effluent of a
gas cylinder, containing 100 ppm
1,3-Butadiene Storage Test Using TBC Coated Charcoal
|
| ||||||||
| storage time (days) |
%
recovery (ambient) |
storage time (days) |
%
recovery (refrigerated) | |||||
|
| ||||||||
| 0 3 6 10 13 17 |
102.2 97.8 100.9 93.8 82.1 81.2 |
102.2 93.3 98.7 87.5 88.8 76.3 |
99.1 93.3 100.9 83.9 80.8 76.8 |
0 4 7 11 14 18 |
97.0 99.5 93.2 98.6 104.1 84.3 |
101.8 96.9 104.9 94.6 96.9 94.6 |
97.8 98.7 102.2 92.4 98.2 96.4 | |
|
| ||||||||
4.6.2. Untreated charcoal
An additional ambient temperature storage test was performed using untreated SKC, Inc. Lot 120 charcoal as sampling media. The test atmosphere was generated and its concentration determined in the same manner as was used for the recommended method. The concentration of the test atmosphere was 1 ppm. The relative humidity of this atmosphere was 70% at 23°C. Sampling was performed at 0.05 L/min for 1 h. The results of this study are presented in Table 4.6.2. and also in Figure 4.6.2.
1,3-Butadiene Ambient Temperature
Storage Test Using SKC, Inc. Lot 120 Charcoal
|
| |||
| storage time days |
% recovery | ||
|
| |||
| 0 3 6 10 13 17 |
33.5 31.3 17.9 29.0 25.2 18.2 |
36.3 30.1 12.8 22.5 23.8 19.8 |
33.5 29.0 14.9 22.6 19.9 17.4 |
|
| |||
4.7. Reproducibility data
Samples were collected from a test atmosphere which was generated
by diluting the effluent of a gas cylinder, containing 100 ppm
Reproducibility
|
| |
| % recovery | statistics |
|
| |
| 100.0 102.9 100.5 98.0 96.6 85.4 |
SD = 6.2 |
|
| |
4.8. A procedure to prepare specially cleaned charcoal coated with TBC
- 4.8.1. Apparatus
- 4.8.1.1. Magnetic stirrer and stir bar.
4.8.1.2. Tube furnace capable of maintaining a temperature of 700°C and equipped with a quartz tube that can hold 30 g of charcoal. A Lindberg Type 55035 tube furnace was used in this evaluation.
4.8.1.3. A means to purge nitrogen gas through the charcoal inside the quartz tube.
4.8.1.4. Water bath capable of maintaining a temperature of 60°C.
4.8.1.5. Miscellaneous laboratory equipment: One-liter vacuum flask, 1-L Erlenmeyer flask, 350-mL Buchner funnel with a coarse fritted disc, 4-oz brown bottle, rubber stopper, Teflon tape, etc.
4.8.2. Reagents
- 4.8.2.1. Phosphoric acid, 10%, by weight, in water. "Baker
Analyzed" Reagent grade was diluted with deionized water for use
in this evaluation.
4.8.2.2. 4-tert-Butylcatechol (TBC). The Aldrich Chemical Company 99% grade was used in this evaluation. CAUTION- The bottle was labeled: Sensitizer! Severe irritant! Toxic! Refrigerate!
4.8.2.3. Specially cleaned coconut shell charcoal, 20/40 mesh. Specially cleaned charcoal (Lot number 482338) was obtained from Supelco, Inc. for use in this evaluation. The cleaning process used by Supelco is proprietary.
4.8.2.4. Nitrogen gas, GC grade.
4.8.3. Procedure
Weigh 30 g of charcoal into a 500-mL Erlenmeyer flask. Add about 250 mL of 10% phosphoric acid to the flask and then swirl the mixture. Stir the mixture for 1 h using a magnetic stirrer. Filter the mixture using a fritted Buchner funnel. Wash the charcoal several times with 250-mL portions of deionized water to remove all traces of the acid. Transfer the washed charcoal to the tube furnace quartz tube. Place the quartz tube in the furnace and then connect the nitrogen gas purge to the tube. Fire the charcoal to 700°C. Maintain that temperature for at least 1 h. After the charcoal has cooled to room temperature, transfer it to a tared beaker. Determine the weight of the charcoal and then add an amount of TBC which is 10% of the charcoal, by weight. CAUTION- TBC is toxic and should only be handled in a fume hood while wearing gloves. Carefully mix the contents of the beaker and then transfer the mixture to a 4-oz bottle. Stopper the bottle with a clean rubber stopper which has been wrapped with Teflon tape. Clamp the bottle in a water bath so that the water level is above the charcoal level. Gently heat the bath to 60°C and then maintain that temperature for 1 h. Cool the charcoal to room temperature and then transfer the coated charcoal to a suitable container.
The coated charcoal is now ready to be packed into sampling tubes. The sampling tubes should be stored in a sealed container to prevent contamination. Sampling tubes should be stored in the dark at room temperature. The sampling tubes should be segregated by coated adsorbent lot number.
4.9. Chromatograms
The chromatograms were obtained using the recommended analytical method. The chart speed was set at 1 cm/min for the first three min and then at 0.2 cm/min for the time remaining in the analysis.
Figures 4.2.2. and 4.9.2 are chromatograms of
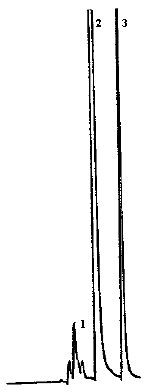
Peak identification was as follows:
1,
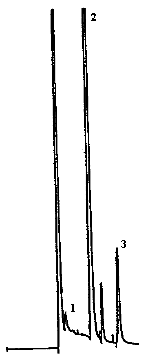
Peak identification was as follows:
1,
collected on TBC coated charcoal.
collected on TBC coated charcoal.
collected on untreated charcoal.
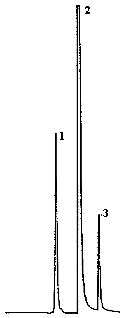
Peak identification was as follows:
1,
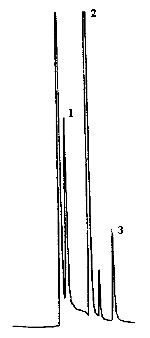
Peak identification was as follows:
1,
5. References
- 5.1. "Current Intelligence Bulletin 41, 1,3-Butadiene", U.S. Dept.
of Health and Human Services, Public Health Service, Center for
Disease Control, NIOSH.
5.2. "NIOSH Manual of Analytical Methods", 2nd ed.; U.S. Dept. of Health Education and Welfare, National Institute for Occupational Safety and Health: Cincinnati, OH. 1977, Vol. 2, Method No. 591 DHEW (NIOSH) Publ. (US), No. 77-157-B.
5.3. Hawley, G. G., Ed. "The Condensed Chemical Dictionary", 8th ed.; Van Nostrand Rienhold Company: New York, 1971; 139.
5.4. Chem. Eng. News (June 10, 1985), (63), 22-66.