| Method no.: | 75 |
| Matrix: | Air |
| Target concentration: | 1.0 ppm (2.56 mg/m3) OSHA PEL |
| Procedure: | Air samples are collected by drawing known volumes of air through glass sampling tubes containing Carbosieve S-III (carbon based molecular sieve) adsorbent. Samples are desorbed with a mixture of 99:1 (v/v) carbon disulfide (CS2)/dimethylformamide (DMF) in the presence of magnesium sulfate. Samples are analyzed by GC using a flame ionization detector. |
| Recommended air volume and sampling rate: |
3 L at 0.05 L/min |
| Reliable quantitation limit: | 0.020 ppm (0.051 mg/m3) |
| Standard error of estimate at the target concentration: (Section 4.7.) |
6.70% |
| Special requirements: | Samples are to be stored at reduced temperature after they have been received at the analytical laboratory. |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: April 1989 | Chemist: Donald Burright |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
In the past, the procedure for monitoring the employee's exposure required that two standard size charcoal tubes, each containing a total of 150 mg of activated charcoal, be used in series for 20 min at 0.05 L/min. The analysis for vinyl chloride was performed on a GC equipped with a flame ionization detector. (Refs. 5.2. and 5.3.)
To eliminate the inconvenience of using two sampling tubes in series, a Carbosieve S-III tube containing 130 mg in the front section and 65 mg in the back section is recommended in this method. This sampling tube also reduces the migration of vinyl chloride during storage to the back section.
Several capillary columns were tested to replace the column
packed with SE-30 on Supelcoport that had been used for many years
to analyze for vinyl chloride. None of the new capillary columns had
as much flexibility to move the vinyl chloride peak as a column
packed with
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
The following information is quoted from a monograph from the International Agency for Research on Cancer.
- Vinyl chloride is a human carcinogen. Its target organs are
the liver, brain, lung and
Epidemiological reports regarding clastogenic effects among vinyl chloride-exposed workers and a single study of increased fetal mortality among the wives of workers who had been exposed to vinyl chloride suggest that vinyl chloride could be mutagenic to humans. Additional support for this suggestion derives from experimental evidence of its mutagenicity. (Ref. 5.4.)
OSHA's health standards for exposure to air contaminants require that an employee's exposure to vinyl chloride not exceed an 8-h time-weighted average of 1.0 ppm (2.56 mg/m3) in workplace air. During any work shift, an employee's exposure may not exceed a ceiling concentration limit of 5 ppm (12.8 mg/m3), averaged over any period not to exceed 15 min. (Ref. 5.1.)
1.1.3. Workplace exposure
Since vinyl chloride is a gas at room temperature and pressure, the common route of toxic exposure is by inhalation. As with many liquidified gases, contact of the skin or eyes with escaping compressed vinyl chloride can produce freezing frostbite. (Ref. 5.5.) An undetermined number of workers are potentially exposed in the workplace to vinyl chloride.
In 1976, over 95% of the vinyl chloride used in the United States was for the production of vinyl chloride homopolymer and copolymer resins. The largest use of polyvinyl chloride resins is in the production of plastic piping and conduit. Other important uses are in floor coverings, in consumer goods, in electrical applications and in transportation applications. (Ref. 5.4.) In 1981, over 3 million tons of vinyl chloride were produced in the United States which had a production capacity of 4.4 million tons. (Ref. 5.6.)
1.1.4. Physical properties and other descriptive information (Ref. 5.6., unless otherwise stated)
| CAS no.: | 75-01-4 |
| molecular weight: | 62.50 |
| molecular formula: | C2H3Cl |
| melting point: | -153.8°C |
| boiling point: | -13.4°C |
| vapor pressure: | 164 kPa (1230 mm Hg) at 0°C 337 kPa (2530 mm
Hg) at |
| vapor density: | 2.2 (air = 1) (Ref. 5.4.) |
| specific gravity: | 0.969 at -14.2/4°C |
| flash point: | -77.75°C (open cup) -78°C (closed cup) (Ref. 5.4.) |
| explosive limit: | 4-22% |
| self-ignition temperature: | 472°C |
| solubility: | slightly soluble in water; soluble in hydrocarbons, oils, chlorinated solvents, and most common organic liquids |
| synonyms: | chloroethylene; chloroethene; ethylene monochloride; VC; VCM |
| structural formula: | 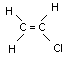 |
The analyte air concentrations throughout this method are based on
the recommended sampling and analytical parameters. Air concentrations
listed in ppm are referenced to
1.2. Limit defining parameters
- 1.2.1. Detection limit of the analytical procedure
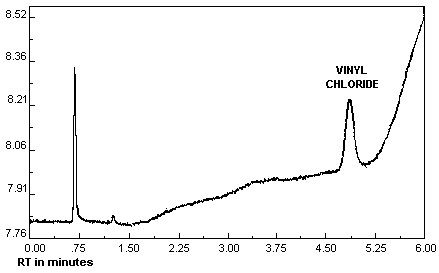
The detection limit of the analytical procedure is 0.760 ng per injection. This is the amount of analyte which gave a vinyl chloride peak whose height is about 9 times the height of a trace contaminant peak. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 0.152 µg per sample (0.051 mg/m3 or 0.020 ppm). This is the amount of analyte spiked on the sampling device which allows recovery of an amount equivalent to the detection limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 0.152 µg per sample (0.051 mg/m3 or 0.020 ppm). This is the smallest amount of analyte spiked on the sampling device which can be quantitated within the requirements of a recovery of at least 75% and a precision (±1.96 SD) of ±25% or better. (Section 4.3.)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of the analyte. When the target concentration of the analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
1.2.4. Instrument response to the analyte
The instrument response over the concentration range of 0.43 to 2 times the target concentration is linear. (Section 4.4.)
1.2.5. Recovery
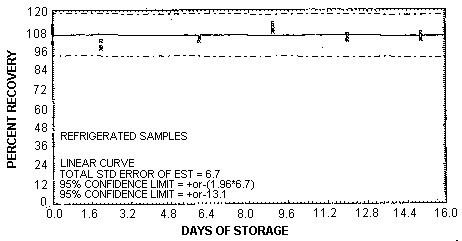
The recovery of vinyl chloride from samples used in the 15-day storage test remained above 103.8% when the samples were stored in a refrigerator at about 4°C. (Section 4.5.) The recovery of an analyte from the collection medium during storage must be 75% or greater.
1.2.6. Precision (analytical procedure only)
The pooled coefficient of variation obtained from replicate injections of analytical standards at 0.43, 1 and 2 times the target concentration is 0.011. (Section 4.6.)
1.2.7. Precision (overall procedure)
The precision at the 95% confidence level for the refrigerated 15-day storage test is ±13.1%. (Section 4.7.) This includes an additional ±5% for sampling error. The overall procedure must provide results at the target concentration that are ±25% or better at the 95% confidence level.
1.2.8. Reproducibility
Six samples, spiked with vinyl chloride, and a draft copy of this procedure were given to a chemist unassociated with this evaluation. The samples were analyzed after 1 day of storage at about 22°C. No individual sample result deviated from its theoretical value by more than the precision reported in Section 1.2.7. (Section 4.8.)
1.3. Advantages
- 1.3.1. This sampling procedure has improved storage stability
over existing coconut shell charcoal samplers due to the lower
migration rate to the back section.
1.3.2. The recommended air volume has been increased to 3 L over the old procedtire's 1-L air volume. This allows for fewer samples to taken and results in lower costs.
1.4. Disadvantages
- 1.4.1. Currently the Carbosieve S-III tube is commercially
available only by special order from Supelco Inc.
1.4.2. The fine mesh size of Carbosieve S-III (60/80) results in a greater pressure drop across the sample tube than occurs with the conventional coconut shell charcoal sampling tube. This results in the need for the 0.05 L/min sampling rate.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. Samples are collected by use of a personal sampling pump
that can be calibrated to within ±5% of the recommended flow rate
with the sampling device attached.
2.1.2. Samples are collected on 4-mm i.d. × 6-mm o.d. × 70 mm sampling tubes packed with two sections of 60/80 mesh Carbosieve S-III. The tubes are packed with 130 mg and 65 mg of adsorbent in the front and back sections respectively. Silanized glass wool plugs are used in the middle and at both ends of the tube to separate and contain the two sections. The sampling tubes are sealed with 7/32 in. plastic caps.
2.2. Reagents
No sampling reagents are required.
2.3. Sampling technique
- 2.3.1. Attach the sampling device to the sampling pump with
plastic tubing such that the large front section of the sampling
tube is exposed directly to the atmosphere. Do not place any tubing
in front of the sampler. Attach the sampler vertically in the
worker's breathing zone in such a manner that it does not impede
work performance or safety.
2.3.2. After sampling for the appropriate time, remove the sampling device and seal the tube with plastic end caps. Wrap each sample end-to-end with a Form OSHA-21 seal.
2.3.3. With each set of samples submit at least one blank sample. Handle the blank sample in the same manner as the other samples except draw no air through it.
2.4. Sampler capacity
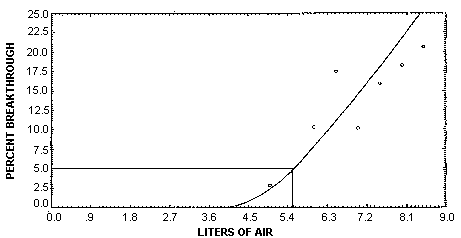
The sampling capacity of the front section of a Carbosieve S-Ill sampling tube was determined by sampling a test atmosphere of 2.57 ppm (6.57 mg/m3) vinyl chloride at ambient temperature and 81% relative humidity. The sampling rate was 0.05 L/min. The 5% breakthrough air volume was 5.3 L. The breakthrough for each air volume tested (2.5 - 8.5 L) was determined by dividing the amount found on the back section by the amount found on the entire tube. (Section 4.9.)
2.5. Desorption efficiency
- 2.5.1. The average desorption efficiency for vinyl chloride over
the range of 0.43 to 2 times the target concentration from
Carbosieve S-III adsorbent was 100.0%. (Section 4.10.)
2.5.2. Desorbed samples, after 24 h, show a loss of about 12.6%. (Section 4.10.)
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 3 L.
2.6.2. The recommended air sampling rate is 0.05 L/min.
2.6.3. When short-term air samples are required, the reliable quantitation limit for a 15-min sample is 0.24 ppm (0.63 mg/m3) at the recommended sampling rate.
2.7. Interferences (sampling)
Suspected interferences should be reported to the laboratory with submitted samples.
2.8. Safety precautions (sampling)
- 2.8.1. The sampling equipment should be attached to the worker
in such a manner that it will not interfere with work performance or
safety.
2.8.2. All safety practices that apply to the work area being sampled should be followed.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A GC equipped with a flame ionization detector (FID). For
this evaluation a
3.1.2. A GC column capable of separating the vinyl chloride peak
from potential interferences. A 10-f t × 1/4-in. o.d. × 2-mm i.d.
glass column packed with 1%
3.1.3. An electronic integrator or other suitable means of
measuring detector response. A
3.1.4. Two-milliliter vials with PTFE-lined caps were used for sample desorption and standard preparation.
3.2. Reagents
- 3.2.1. Vinyl chloride. The vinyl chloride used in this
evaluation to prepare standards was 99.9% pure. The concentration of
the vinyl chloride cylinder used to generate a dynamic test
atmosphere was 101 ppm in nitrogen. Both tanks were purchased from
Alphagaz Liquid Air (Walnut Creek, CA).
3.2.2. Carbon disulfide. Reagent grade or better
CS2 should be used. In this evaluation,
3.2.3. Dimethylformamide Reagent grade or better should be used.
3.2.4. Magnesium sulfate, anhydrous powder, is used as a drying agent. The magnesium sulfate used in this evaluation was purchased from Aldrich Chemical (Milwaukee, WI).
3.2.5. Desorbing solution. This consists of a solution of 99:1 (v/v) benzene-free CS2/dimethylformamide. An internal standard such as n-heptane can be used.
3.2.6. n-Heptane. This was used as the internal standard in the desorbing solution. The solution is prepared by adding 5 µL of n-heptane to 1 L of desorbing solution. The n-heptane was purchased from Burdick & Jackson (Muskegon, MI).
3.3. Standard preparation
- 3.3.1. Prepare analytical standards by injecting microliter
amounts of pure vinyl chloride gas into capped 2-mL vials containing
130 mg of Carbosieve S-III adsorbent. Force the vinyl chloride gas
from the syringe with the opening of the needle under the adsorbent.
A standard containing 7.69 µg/mL (at 23°C and 650 mm Hg) could be
prepared by injecting 3.5 µL of vinyl chloride onto the adsorbent.
Analytical standards can also be prepared by bubbling vinyl chloride gas from a syringe into a vial containing desorbing solution. This procedure was used to determine the desorption efficiency and was not used for the rest of the tests.
3.3.2. The mass of vinyl chloride gas which was used to prepare standards can be determined by use of the following equations:
MV = (22.41)(760/BP)(273+T)/(273)
| where | MV BP T |
= = = |
ambient molar volume, L/mole ambient barometric pressure, mm Hg ambient temperature, °C |
µg/µL = 62.50/MV
µg = (µg/µL)(µL of vinyl chloride injected)(purity)
3.3.3. Wait for 30 min and uncap the vial. Add 1.0 mL of desorbing solution to the vial and immediately recap it.
3.3.4. Shake the vials vigorously by hand several Mmes during the next 30 min.
3.3.5. Prepare at least three standards to generate a calibration curve. Ensure that the amount of vinyl chloride found in the samples is within the range of the standards. Prepare additional standards if necessary.
3.4. Sample preparation
- 3.4.1. Remove the plastic caps from the sample tube and
carefully transfer each section of the adsorbent to separate vials.
3.4.2. Add approximately 150 mg of anhydrous magnesium sulfate powder to each sample.
3.4.3. Add 1.0 mL of desorbing solution to each vial and seal the vials with PTFE-lined caps.
3.4.4. Shake the vials vigorously by hand several times during the next 30 min.
3.5. Analysis
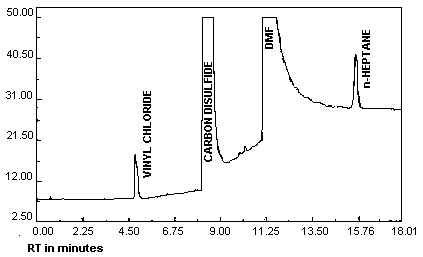
- 3.5.1. Analytical conditions
| GC conditions | |
| temperatures: | 40°C (column) 200°C (injector) 220°C (detector) |
| temp program: | hold initial temp 1.0 min, increase temp at 5°C/min to 60°C, then increase temp at 50°C/min to 240°C, hold temp for 10 min |
| column gas flow: | 30 mL/min (nitrogen) |
| septum purge: | 1.5 mL/min (nitrogen) |
| injection size: | 5.0 µL |
| column: | 10-ft × 1/4-in. o.d. × 2-mm i.d. glass column
containing 1% |
| retention times: | 4.8 min (vinyl chloride) 15.5 min (n-heptane) |
| FID conditions | |
| hydrogen flow: | 30 mL/min |
| air flow: | 450 mL/min |
| chromatogram: | Figure 3.5.1. |
3.5.2. Measure detector response using a suitable method such as electronic integration.
3.5.3. Use an internal standard (ISTD) procedure to prepare a calibration curve using several freshly prepared standards over a range of concentrations. Bracket the samples with analytical standards.
3.6. Interferences (analytical)
- 3.6.1. Any compound having a similar retention time as the
analyte is a potential interference. Generally, chromatographic
conditions can be altered to separate an interference from the
analyte.
3.6.2. Retention time on a single column is not proof of chemical identity. Analysis by an alternate GC column or confirmation by mass spectrometry are additional means of identification.
3.7. Calculations
- 3.7.1. When an ISTD method is used, the vinyl chloride
concentration of an analytical standard is determined by the
following equation.
Ca = (Aa)(Ra)(Ci)/(Ai)(Ri)
| where | C A R a i |
= = = = = |
concentration area or peak height response factor analyte internal standard |
3.7.2. Prepare calibration curves by plotting the vinyl chloride concentration determined from an ISTD method versus the theoretical analytical standard concentrations. Determine the best-fit line through the data points.
3.7.3. Determine the concentration of a sample by comparing its concentration determined by the ISTD method to the calibration curve. Perform blank corrections for each section before adding the results together. Add any amount of vinyl chloride found on the back section to the amount found on the front section.
3.7.4. The air concentration of vinyl chloride can be expressed in mg/m3 by using the following equation:
mg/m3 = (A)(B)/(C)
| where | A B C |
= = = |
µg/mL of vinyl chloride from Section 3.7.2. desorption volume (1 mL) liters of air sampled |
No desorption correction is required because standards are prepared in the presence of Carbosieve S-III adsorbent.
3.7.5. Convert vinyl chloride results in mg/m3 to ppm using the following equation:
ppm = (mg/m3)(24.46)/(62.50)
| where | mg/m3 24.46 62.50 |
= = = |
results from 3.7.3. molar volume at 760 mm Hg and 25°C molecular weight of vinyl chloride |
3.8. Safety precautions (analytical)
- 3.8.1. CAUTION. VINYL CHLORIDE IS OR SHOULD BE CONSIDERED
CARCINOGENIC TO HUMANS. Restrict use of pure compounds and
concentrated standards to regulated areas. Avoid skin contact and
inhalation of all chemicals.
3.8.2. Restrict the use of all chemicals to a fume hood if possible.
3.8.3. Wear safety glasses and a lab coat at all times while in the laboratory areas.
4. Backup Data
- 4.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 0.760 ng per injection, based on a 5.0-µL injection of a 0.152 µg/mL standard. This amount produced a vinyl chloride peak whose height is about 9 times the height of a trace contaminant peak in the chromatogram. A chromatogram of the detection limit of the analytical procedure is shown in Figure 4.1.
4.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 0.152 µg per sample (0.051 mg/m3 or 0.020 ppm). The injection size recommended in the analytical procedure (5.0 µL) was used in the determination of the detection limit of the overall procedure. Eight vials containing 130 mg of Carbosieve S-III resin were spiked with a syringe containing 0.152 µg of pure vinyl chloride gas. The samples were desorbed about 1 h after being spiked.
Detection Limit of the Overall
Procedure
|
| ||
| sample | theoretical amount | amount recovered |
| number | (µg) | (µg) |
|
| ||
| 1 | 0.152 | 0.128 |
| 2 | 0.152 | 0.146 |
| 3 | 0.152 | 0.157 |
| 4 | 0.152 | 0.129 |
| 5 | 0.152 | 0.147 |
| 6 | 0.152 | 0.119 |
| 7 | 0.152 | 0.155 |
| 8 | 0.152 | 0.118 |
|
| ||
4.3. Reliable quantitation limit data
The reliable quantitation limit is 0.152 µg per sample (0.051 mg/m3 or 0.020 ppm). The injection size recommended in the analytical procedure (5.0 µL) was used in the determination of the reliable quantitation limit. Eight vials containing 130 mg of Carbosieve S-III resin were vapor-spiked with 0.152 µg of vinyl chloride. Because the recovery of vinyl chloride from the spiked samples was greater than 75% and had a precision of ±25 or better, the detection limit of the overall procedure and reliable quantitation limit are the same.
Reliable Quantitation Limit
(Based on samples and data of Table 4.2.)
|
| |||
| percent recovered |
statistics | ||
|
|
|||
| 84.2 | |||
| 96.1 | = | 90.4 | |
| 103.3 | SD | = | 10.4 |
| 84.9 | Precision | = | (1.96)(±10.4) |
| 96.7 | = | ±20.4 | |
| 78.3 | |||
| 102.0 | |||
| 77.6 | |||
|
| |||
4.4. Instrument response to vinyl chloride
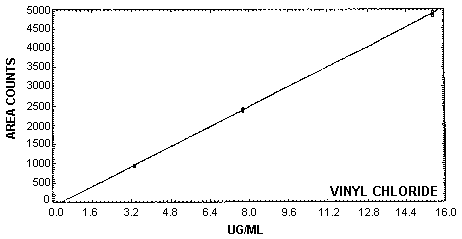
The instrument response to vinyl chloride over the range of 0.43 to 2 times the target concentration is linear with a slope of 322 area counts per microgram per milliliter. The precision of the response to vinyl chloride was determined by multiple injections of vinyl chloride standards. The data below is presented graphically in Figure 4.4.
Instrument Response to Vinyl
Chloride
|
| |||
| × target conc. | 0.43× | 1× | 2× |
| µg/sample | 3.32 | 7.75 | 15.51 |
|
| |||
| area counts | 975.3 | 2444.4 | 4899.4 |
| 963.3 | 2440.1 | 4962.5 | |
| 961.8 | 2419.5 | 4939.7 | |
| 962.5 | 2416.2 | 4854.8 | |
| 955.3 | 2374.2 | 4851.1 | |
| 946.9 | 2374.5 | 4840.8 | |
| 960.9 | 2411.5 | 4891.4 | |
|
| |||
4.5. Storage data
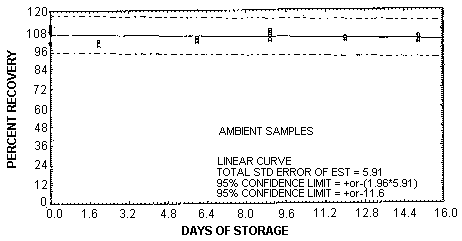
Forty-eight storage samples were collected by sampling a dynamic-ally generated atmosphere containing 4.95 mg/m3 or 1.94 ppm of vinyl chloride and 81% relative humidity for 30 min at 0.05 L/min. One-half of the tubes was stored in a refrigerator (4°C) and the other half was stored in a closed drawer at ambient temperature (about 22°C). At 2-4 day intervals, four samples were selected from each of the two storage sets and analyzed. The results are listed below and shown graphically in Figures 4.5.1. and 4.5.2.
Storage Test
|
| ||||||||
| storage time | % recovery | % recovery | ||||||
| (days) | (ambient) | (refrigerated) | ||||||
|
|
|
| ||||||
| 0 | 110.9 | 108.5 | 106.8 | 110.4 | 110.9 | 108.5 | 106.8 | 110.4 |
| 106.7 | 108.1 | 109.2 | 100.1 | 106.7 | 108.1 | 109.2 | 100.1 | |
| 2 | 101.1 | 98.8 | 101.3 | 101.3 | 97.2 | 96.4 | 96.2 | 101.3 |
| 6 | 103.1 | 101.5 | 100.9 | 102.1 | 103.7 | -- | 101.4 | 102.8 |
| 9 | 105.3 | 101.6 | 106.4 | 107.7 | 112.0 | 110.4 | 107.5 | 106.8 |
| 12 | 103.0 | 102.3 | 101.6 | 103.3 | 101.7 | 103.6 | 104.8 | 101.5 |
| 15 | 100.5 | 103.8 | 102.7 | 102.8 | 101.6 | 102.8 | 105.6 | 102.9 |
|
| ||||||||
The analysis of the back sections of the samples generated for the storage test indicate that the samples do not have to be shipped at reduced temperature but should be stored at reduced temperature because the vinyl chloride starts to migrate to the back section after 9 days of storage at ambient temperature. The average amount of vinyl chloride found on the back sections was 2.5% after 12 days of storage and 5% after 15 days. No vinyl chloride was found on any of the refrigerated back sections.
4.6. Precision (analytical method only)
The precision of the analytical procedure is defined as the pooled coefficient of variation determined from replicate injections of vinyl chloride standards at 0.43, 1 and 2 times the PEL. Based on the data of Table 4.4., the coefficients of variation (CV) for the three levels and the pooled coefficient of variation (CV) were calculated and are listed below.
Precision of the Analytical Method
(Based on the Data of Table 4.4.)
|
| |||
| × target conc. | 0.43× | 1× | 2× |
| µg/sample | 3.32 | 7.75 | 15.5 |
|
| |||
| SD1 | 9.4 | 30.8 | 50.9 |
| CV | 0.0098 | 0.0128 | 0.0104 |
|
| |||
| 1 standard deviation is in area counts | |||
4.7. Precision (overall procedure)
The precision of the overall procedure is determined from the storage data. The determination of the standard error of estimate (SEE) for a regression line plotted through the graphed storage data allows the inclusion of storage time as one of the factors affecting overall precision. The SEE is similar to the standard deviation except it is a measure of dispersion of data about a regression line instead of about a mean. It is determined with the following equation:
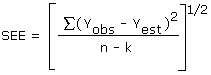
| where | ||
| n | = | total no. of data points |
| k | = | 2 for a linear regression |
| k | = | 3 for a quadratic regression |
| Yobs | = | observed % recovery at a given time |
| Yest | = | estimated % recovery from the regression line at the same given time |
An additional 5% for pump error is added to the SEE by the addition
of variances. The precision at the 95% confidence level is obtained by
multiplying the SEE (with pump error included) by 1.96 (the
4.8. Reproducibility data
Six samples, collected from a dynamically generated atmosphere containing vinyl chloride, were given to a chemist unassociated with this study. The samples were analyzed after being' stored for 1 day at 22°C. No sample result had a percent deviation greater than the precision of the overall procedure, which is ±13.1%.
Reproducibility Data
|
| |||
| µg spiked | µg recovered | % recovered | % deviation |
|
| |||
| 6.36 | 7.11 | 111.8 | +11.8 |
| 6.36 | 6.63 | 104.3 | +4.3 |
| 6.36 | 6.96 | 109.4 | +9.4 |
| 6.36 | 6.57 | 103.3 | +3.3 |
| 6.36 | 6.84 | 107.6 | +7.6 |
| 6.36 | 6.45 | 101.4 | +1.4 |
|
| |||
4.9. Sampler capacity
Sampler capacity was determined by sampling from a dynamically generated atmosphere of about 2.57 ppm (6.57 mg/m3) vinyl chloride with whole Carbosieve S-III sampling tubes. The relative humidity of the test atmosphere was 81%. The percentage of breakthrough was calculated by dividing the amount found on the back section by the amount found on the entire tube. This percentage was plotted versus the air volume (Figure 4.9.) to determine the five percent breakthrough air volume of 5.6 L. These samples were collected over 2 days from different dynamically generated test atmospheres.
Breakthrough on the Carbosieve S-III Tube
|
| ||||
| air volume | front section | back section | total | breakthrough |
| (L) | (µg) | (µg) | (µg) | (%) |
|
| ||||
| 2.5 | 15.73 | 0 | 15.73 | 0 |
| 3.5 | 20.25 | 0 | 20.25 | 0 |
| 4.0 | 25.47 | 0 | 25.47 | 0 |
| 4.5 | 29.13 | 0 | 29.13 | 0 |
| 5.0 | 34.32 | 1.05 | 35.37 | 2.97 |
| 5.5 | 35.82 | 1.01 | 36.83 | 2.74 |
| 6.0 | 34.42 | 4.04 | 38.46 | 10.5 |
| 6.5 | 38.19 | 8.17 | 46.36 | 17.6 |
| 7.0 | 42.09 | 4.85 | 46.94 | 10.3 |
| 7.5 | 43.09 | 8.27 | 51.36 | 16.1 |
| 8.0 | 43.41 | 9.80 | 53.21 | 18.4 |
| 8.5 | 43.78 | 11.48 | 55.26 | 20.8 |
|
| ||||
4.10. Desorption efficiency and stability of desorbed samples
- 4.10.1. Desorption efficiency
The desorption efficiency (DE) of vinyl chloride was determined by vapor-spiking 130-mg portions of Carbosieve S-III adsorbent with vinyl chloride at 0.43 to 2 times the target concentration. These samples were stored overnight and then desorbed with desorbing solution and analyzed. The average desorption efficiency over the studied range was 100.0%. The analytical standards used to analyze the desorption efficiency samples were made in vials containing desorbing solution that had been vapor-spiked with vinyl chloride.
Desorption Efficiency of Vinyl Chloride
|
| |||
| × target conc. | 0.43× | 1× | 2× |
| µg/sample | 3.33 | 7.77 | 15.6 |
|
| |||
| DE, % | 95.4 | 100.8 | 99.4 |
| 99.2 | 100.4 | 102.2 | |
| 104.9 | 98.4 | 98.2 | |
| 103.1 | 101.3 | 101.0 | |
| 97.9 | 98.3 | 100.9 | |
| 98.9 | 99.4 | 98.9 | |
| 101.1 | 101.4 | 100.8 | |
| 98.7 | 101.5 | 98.2 | |
| 99.9 | 100.2 | 100.0 | |
|
| |||
4.10.2. Stability of desorbed samples
The stability of desorbed samples was investigated by reanalyzing
storage test samples, from Day 6, 24 h after initial analysis. The
original analysis had been per formed overnight and the vials were
recapped the next morning. The samples were reanalyzed with fresh
standards. The average recovery, compared to the average recovery of
the original analysis, was 88.7% or a
Stability of Desorbed Samples
|
| ||
| initial recovery | recovery after 24 h | percent |
| (percent) | (percent) | change |
|
| ||
| 103.1 | 90.3 | -12.8 |
| 101.5 | 86.1 | -15.4 |
| 100.9 | 87.7 | -13.2 |
| 102.1 | 89.6 | -12.5 |
| 103.7 | 90.6 | -13.1 |
| 101.4 | 89.5 | -11.9 |
| 102.8 | 86.8 | -16.0 |
|
| ||
0.760 ng per injection.
slope = 322 area counts per micrograms per milliliter.
5. References
- 5.1. Code of Federal Regulations, Title 29;
1910.1017, Washington, D.C., 1987, pp. 784-790.
5.2. "NIOSH Manual of Analytical Methods", 3rd ed.; U.S. Department of Health and Human Services, Center for Disease Control, NIOSH; Cincinnati, OH, 1984, Vol. 2, Method 1007, DHHS (NIOSH) Publ. No. 84-100.
5.3. "OSHA Analytical Methods Manual"; U.S. Department of Labor, Occupational Safety and Health Administration; OSHA Analytical Laboratory: Salt Lake City, UT, 1985; Method 4; American Conference of Government Industrial Hygienists (ACCIH): Cincinnati, ISBN: 0-936712-66-X.
5.4. International Agency for Research on Cancer, "IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans: Some Monomers, Plastics and Synthetic Elastomers, and Acrolein", IARC, Lyon, 1979, Vol. 19, pp. 377-419.
5.5. "Documentation of the Threshold Limit Values and Biological Indices", 5th ed.; American Conference of Government Industrial Hygienists (ACCIH): Cincinnati, ISBN: 0-036712-68-6, 1986; pp. 623-626.
5.6. Cowfer, J.A.; Magistro, A.J. in "Kirk-Othmer Encyclopedia of Chemical Technology"; 3rd ed.; Crayson, M., Ed.; John Wiley & Sons, New York, 1983, Vol. 23, pp. 865-884.