1. General Discussion
1.1 Background
1.1.1 History
The current
NIOSH method (Ref. 5.1) for monitoring airborne acetic anhydride
specifies collection with a midget bubbler containing alkaline
hydroxylamine and analysis by spectrophotometry. The bubbler is
cumbersome and spectrophotometry is nonspecific. Airborne acetic
anhydride has also been collected on Porapak N solid sorbent and
analyzed by GC/FID (Ref. 5.2). However, the samples can not be stored
for more than 10 days without significant degradation, even with
freezing. A more convenient sampling method using glass beads coated
with 1-2PP has been reported (Ref. 5.3). The derivative,
1-acetyl-4-(2-pyridyl)piperazine (AcPP), was analyzed by high
performance thin layer chromatography. The derivatization stabilizes
the analyte and eliminates the interference from acetic acid. It is
recognized that ketene and acetyl chloride will also react with 1-2PP
to form AcPP, but neither chemical is used widely in industry (Ref.
5.4).
In this method, a glass fiber filter (GFF) impregnated
with 1-2PP was selected as the sampling medium and the derivative
(AcPP) was analyzed by GC/NPD. Analysis by HPLC/UV is also possible,
but not as sensitive as GC/NPD. A flow rate of 0.05 L/min was selected
because capacity of the sampler was reduced at higher flow rates.
Samples are collected closed-face to minimize contamination. The
problem of the analyte concentrating in the center of the filter is
minimized by the use of an extra spacer in the
cassette.
1.1.2 Toxic effects (This section is for
information only and should not be taken as the basis of OSHA policy.)
(Ref. 5.5 and 5.6)
Acetic anhydride vapors may be irritating to
the eyes, nose and throat. Inhalation of vapors may cause severe
irritation of the respiratory system. Due to its irritating effects, a
ceiling value of 5 ppm (20 mg/m3) is set for OSHA PEL.
Contact with the skin or eyes may cause burns. Ingestion may cause
severe burns of the mouth, throat, and stomach. Ingestion may also
cause nausea and vomiting.
1.1.3 Workplace exposure
Exposure to acetic anhydride may occur in the following
operations: manufacture of cellulose esters, fibers, plastics,
lacquers, protective coating solutions, photographic films, cigarette
filters, magnetic tape, and thermoplastic molding compositions;
manufacture of pharmaceuticals and pharmaceutical intermediates; use
in organic synthesis as an acetylating agent, bleaching agent, and
dehydrating agent; synthesis of perfume chemicals, explosives, and
weed killers; use in acetylation of animal and vegetables oils; use as
an acetylating agent and dehydrating agent in textile dyeing, chemical
treatment of paper, and chemical analysis. (Ref. 5.7) Of these, by far
the greatest single application for acetic anhydride is in the
manufacture of cellulose esters. It is estimated that 95% of the total
U.S. production is used for this purpose. (Ref.
5.8)
1.1.4 Physical properties and other descriptive
information (Ref. 5.9. unless noted otherwise)
| chemical name: |
acetic anhydride |
| CAS no.: |
108-24-7 |
| synonyms: |
acetic acid, anhydride; acetic
oxide; acetyl anhydride; acetyl ether; acetyl oxide; ethanoic
anhydrate |
| formula: |
(CH3CO)2O |
| mol wt: |
102.10 |
| boiling point: |
139°C |
| melting point: |
-73°C |
| vapor pressure: |
0.67 kPa (5 mmHg) at 25°C
(Ref.5.10) |
| flash point: |
49°C (closed cup) |
| specific gravity: |
1.080 |
| color: |
colorless |
| odor: |
strong acetic odor |
| |
|
| Derivative: |
(Ref. 5.11) |
| chemical name: |
1-acetyl-4-(2-pyridyl)piperazine |
| structural formula: |
Figure
1.1.4 |
| mol wt: |
205.27 |
| melting point: |
89.5-91.5°C |
| solubility: |
soluble in methanol, acetonitrile,
toluene, isopropanol, methylene chloride |
| mass spectrum: |
Figure
1.1.4 |
The analyte air concentrations throughout this
method are based on the recommended sampling and analytical parameters.
Air concentrations listed in ppm are referenced to 25°C and 101 kPa (760
mmHg). The analyte concentrations are listed as those of acetic
anhydride even though the derivative is the actual species
analyzed.
1.2 Limit defining parameters
1.2.1 Detection limit of the
analytical procedure
The detection limit of the analytical
procedure is 11 pg on column (1.0 µL injection of 0.076 µg/mL solution
with 7:1 split). This is the amount of analyte which gave a peak with
height about 5 times the baseline noise. (Section
4.1)
1.2.2 Detection limit of the overall
procedure
The detection limit of the overall procedure is 0.51
µg per sample (0.16 ppm, 0.68 mg/m3). This is the amount of
analyte spiked on the sampling device which allows recovery of an
amount equivalent to the detection limit of the analytical procedure.
(Section 4.2)
1.2.3 Reliable quantitation
limit
The reliable quantitation limit is 0.51 µg per sample
(0.16 ppm, 0.68 mg/m3). This is the smallest amount of
analyte spiked on the sampling device which can be quantitated within
the requirements of a recovery of at least 75% and a precision (±1.96
SD) of ±25% or better. (Section 4.3)
The reliable quantitation limit and detection
limits reported in the method are based upon optimization of the
instrument for the smallest possible amount of the analyte. When the
target concentration of the analyte is exceptionally higher than these
limits, they may not be attainable at the routine operating
parameters.
1.2.4 Instrument response to the
analyte
The instrument response over the concentration range of
0.5 to 2 times the target concentration is linear. (Section
4.4)
1.2.5 Recovery
The recovery of AcPP from
samples used in a 15-day storage test remained above 83% when the
samples were stored at ambient temperature. (Section 4.5) The recovery
of an analyte from the collection medium during storage must be 75% or
greater.
1.2.6 Precision (analytical procedure
only)
Nine analytical standards were prepared from three stock
standards (individually prepared) by making serial dilutions to
represent 0.5, 1, and 2 times the target concentration.
The pooled
coefficient of variation obtained from duplicate injections of the
nine analytical standards is 0.034. (Section 4.6)
1.2.7
Precision (overall procedure)
The precision at the 95%
confidence level for the ambient 15-day storage test is ±14.7%.
(Section 4.7) This includes an additional ±5% for pump error. The
overall procedure must provide results at the target concentration
that are ±25% or better at the 95% confidence
level.
1.2.8 Reproducibility
A draft copy of this
procedure and six samples collected from a controlled test atmosphere
at the target concentration [80% RH, 23°C, 87.7 kPa (658 mmHg)] were
given to a chemist unassociated with this evaluation. The samples were
stored in a refrigerator at 0°C for 1 day before being analyzed. No
individual sample result deviated from its theoretical value by more
than the precision reported in Section 1.2.7. (Section
4.8) 1.3
Advantage
The acetic anhydride is derivatized in situ,
eliminating the possibility of its being hydrolyzed during
storage.
1.4 Disadvantage
The method is subject to
interference from ketene and acetyl chloride. 2. Sampling Procedure
2.1 Apparatus
2.1.1 A personal sampling pump
that can be calibrated to within ±5% of the recommended flow rate with
the sampling device in line.
2.1.2 A four-piece
polystyrene cassette containing two glass fiber filters each
impregnated with 2.5 mg of 1-2PP. Impregnated filters are prepared by
applying 0.5 mL of a solution of 5.0 mg/mL 1-2PP in methylene chloride
to each glass fiber filter and allowing them to dry in a hood.
Impregnated filters should be stored in a closed jar at reduced
temperature before assembly.
2.1.3 Assemble the cassette
by placing the extra spacer in front of the first filter. (Figure
2.3.1) 2.2
Reagents
No reagent is required for sampling.
2.3
Sampling technique
2.3.1 Remove the plugs from the
top and bottom pieces. Attach the sampler to the sampling pump with a
piece of flexible tubing and place it in the worker's breathing
zone.
2.3.2 Replace the small plugs after sampling. Seal
the sample end-to-end with an official OSHA seal (Form
21).
2.3.3. Submit at least one blank with each set of samples.
Handle the blank the same as the other samples except draw no air
through it.
2.3.4 List any potential interferences on the
sample data sheet. 2.4
Sampler capacity
The sampler capacity was evaluated with a test
atmosphere (80% RH) at 1.7 times the target concentration. Two samplers
were placed in series. The upstream sampler contained only the front
filter. The back sampler was replaced with a new sampler every 20 min to
monitor the downstream air concentration. The 5% breakthrough point,
defined as the point where the downstream analyte concentration is 5% of
the upstream concentration, was reached at 180 min of sampling at the
recommended sampling rate. (Section 4.9)
2.5 Extraction
efficiency and stability of extracted samples (Section 4.10)
2.5.1 The average extraction
efficiency at the target concentration was 97.7%.
2.5.2
Extracted samples remain stable for at least 24 h when stored at room
temperature. 2.6
Recommended air volume and sampling rate
2.6.1 The recommended air volume
is 0.75 L.
2.6.2 The recommended air sampling rate is
0.05 L/min. 2.7
Interferences (sampling)
Compounds that can react with 1-2PP,
such as isocyanates, acid chlorides, and other anhydrides, may interfere
by consuming part of the derivatizing agent. Acetyl chloride and ketene
cause positive interferences.
2.8 Safety precautions
(sampling)
Attach the sampling equipment to the worker in such a
manner that it will not interfere with work performance or safety.
Follow all safety practices applicable to the work
area. 3. Analytical Procedure
3.1 Apparatus
3.1.1 A GC equipped with a
nitrogen-phosphorus detector (NPD). A Hewlett-Packard 5890 GC equipped
with an NPD and a 7673A autosampler was used in this
evaluation.
3.1.2 A GC column capable of separating AcPP,
benzalazine, and any interferences. A 15 m SPB-5 (0.32-mm i.d., 1-µm
film) column was used in this evaluation.
3.1.3 An
electronic integrator or other suitable means of measuring detector
response. A Hewlett-Packard 3357 laboratory data system was used in
this evaluation.
3.1.4 Scintillation vials, 20
mL.
3.1.5 Volumetric flasks and pipets.
3.2 Reagents
3.2.1 Acetic anhydride. Acetic
anhydride, ACS reagent grade, was obtained from Aldrich
Chemical.
3.2.2 1-(2-Pyridyl)piperazine.
1-(2-Pyridyl)piperazine, 98%, was obtained from Aldrich
Chemical.
3.2.3 1-Acetyl-4-(2-pyridyl)piperazine (AcPP).
Synthesized as in Section 4.12.
3.2.4 Benzalazine.
Benzalazine from K & K was used in this evaluation.
3.2.5 Toluene. Toluene was obtained from American
Burdick and Jackson.
3.2.6 2-Propanol. 2-Propanol,
Optima, from Fisher was used.
3.2.7 Extraction solvent
with internal standard. Dissolve 10 mg of benzalazine in 1 L of
toluene/2-propanol (50:50). 3.3 Standard preparation
3.3.1 Prepare stock standards by
weighing 10-20 mg of AcPP (prepared as in Section 4.12.) in 10-mL
volumetric flasks and diluting to volume with the extraction solvent.
Apply a factor of 0.4973 to the weight of AcPP to convert it to that
of free acetic anhydride. For example, 10 mg of AcPP dissolved in 10
mL will give a standard stock solution representing 0.4973 mg/mL or
497.3 µg/mL of acetic anhydride.
(MW acetic anhydride) /( MW
AcPP) = 102.09/205.27 = 0.4973
3.3.2 Prepare
analytical standards by further diluting the stock standards with the
extraction solvent. An analytical standard of 3.0 µg/mL represents 1
times the target concentration.
3.3.3 Prepare a
sufficient number of standards to generate calibration curves.
Analytical standard concentrations must bracket sample concentrations.
3.4 Sample
preparation
3.4.1 Transfer the front and the
back filters into separate 20-mL scintillation vials.
3.4.2
Add 5.0 mL of the extraction solvent to each vial.
3.4.3 Cap
the vials and shake them on a mechanical shaker for 1 h.
3.5 Analysis
3.5.1 GC conditions
| column: |
SPB-5 (15 m, 0.32-mm i.d.,
1-µm film) |
| zone temperatures: |
column - |
170°C to 250°C at 10°C/min |
| |
injector - |
200°C |
| |
detector - |
250°C |
| gas flows (mL/min): |
hydrogen (carrier) - |
3.0 |
| |
nitrogen - |
27 |
| |
air - |
95 |
| |
hydrogen - |
3.8 |
| injection volume: |
1 µL (with a 7:1 split) |
|
| retention times (min): |
1-2PP - |
1.73 |
| |
benzalazine - |
4.27 |
| |
AcPP - |
4.64 |
| chromatogram: |
Figure
3.5.1 |
|
3.5.2 Measure detector response using a suitable
method such as electronic integration.
3.5.3 Construct a
calibration curve using an internal standard method by plotting µg/mL
versus ISTD-corrected response of standard injections. Bracket the
samples with analytical standards. 3.6 Interferences (analytical)
3.6.1 Any compound that responds
on an NPD and has a similar retention time as benzalazine or AcPP is a
potential interference. Generally, chromatographic conditions can be
altered to separate an interference.
3.6.2 Ketene and
acetyl chloride, being able to react with 1-2PP to form AcPP, are
interferences.
3.6.3 Retention time on a single column is
not considered proof of chemical identity. Analyte identity should be
confirmed by GC/mass spectrometry if possible. The mass spectrum of
AcPP is shown in Figure 1.1.3. 3.7 Calculations
The analyte concentration
for samples is obtained from the calibration curve in terms of
micrograms per milliliter uncorrected for extraction efficiency. The
concentrations are converted to µg per sample by multiplying with 5.0 mL
(extraction volume). The back filter is analyzed primarily to determine
if there was any breakthrough from the front filter during sampling. If
a significant amount of analyte is found on the back filter (e.g.,
greater than 25% of the amount found on the front filter), this fact
should be reported with sample results. If any analyte is found on the
back filter, it is added to the amount found on the front filter. This
total analyte amount is corrected by subtracting the amount found in the
blank. The air concentration is obtained by using the following
formula.
| mg/m3 = |
(micrograms of analyte per sample)
(liters of air sampled) (extraction
efficiency) |
where
extraction efficiency = 0.977
| ppm = |
(mg/m3) (24.46)
(102.09) |
| where: |
24.46 = molar volume (liters) at
101 kPa (760 mmHg) and 25°C
102.09 = molecular weight of acetic
anhydride |
3.8
Safety precautions (analytical)
Avoid skin contact and inhalation
of all chemicals. Restrict the use of all chemicals to a fume hood when
possible. Wear safety glasses and a lab coat at all times while in the
lab area. 4 Backup Data
4.1 Detection limit of the
analytical procedure
The detection limit of the analytical
procedure is 11 pg on column (1.0 µL injection of 0.076 µg/mL solution
with 7:1 split). This is the amount of analyte that will give a peak
with height approximately 5 times the height of the baseline noise. A
chromatogram of the detection limit of the analytical procedure is shown
in Figure
4.1.
4.2 Detection limit of the overall procedure
The detection limit of the overall procedure is 0.51 µg per
sample (0.16 ppm, 0.68 mg/m3). This is the amount of analyte
spiked on the sampling device which allows recovery of an amount
equivalent to the detection limit of the analytical procedure. Six
1-2PP-impregnated glass fiber filters were each liquid spiked with 0.51
µg of acetic anhydride (6 µL of 85.5 µg/mL solution). The samples were
extracted 24 h later with 5.0 mL of the extraction solvent. The
injection size listed in the analytical procedure (1 µL) was used in the
determination of the detection limit of the overall
procedure.
Table 4.2
Detection Limit of the Overall Procedure |
|
sample
number |
theoretical
amount
(µg) |
amount
recovered
(µg) |
|
1
2
3
4
5
6 |
0.513
0.513
0.513
0.513
0.513
0.513 |
0.577
0.535
0.578
0.546
0.587
0.500 |
|
4.3 Reliable
quantitation limit
The reliable quantitation limit is also 0.51
µg per sample (0.16 ppm, 0.68 mg/m3). This was derived from
the samples and data of Table 4.2. Because the recovery was greater than
75% and the precision (1.96 SD) was less than 25%, the detection limit
of the overall procedure and reliable quantitation limit are the same.
Table
4.3
Reliable Quantitation Limit
(based on samples and data
of Table 4.2)
|
| % recovery |
statistics |
|
| 112.5 |
|
| 104.3 |
 = = |
108.0% |
| 112.7 |
SD = |
6.5% |
| 106.4 |
Precision = |
±(1.96)(6.5%) |
| 114.4 |
= |
±12.7% |
| 97.5 |
|
|
4.4 Instrument
response
The instrument response (ISTD corrected) to AcPP over
the range of 0.5 to 2 times the target concentration is linear with a
slope of 1.055. The responses to AcPP were determined by duplicate
injections of nine analytical standards prepared from three stock
standards (individually prepared). Because the concentrations of these
standards were slightly different, the ratios of response to µg/mL were
compared. The data are summarized in Table 4.4. and presented
graphically in Figure
4.4.
Table
4.4
ISTD-corrected Instrument Response to AcPP
|
0.5×
target
µg/mL response ratio1 |
1×
target
µg/mL response ratio |
2×
target
µg/mL response ratio |
|
1.552
1.552
1.452
1.452
1.432
1.432 |
1.295
1.269
1.260
1.192
1.134
1.131 |
0.8344
0.8177
0.8678
0.8209
0.7919
0.7898 |
3.103
3.103
2.904
2.904
2.865
2.865 |
2.835
2.811
2.669
2.651
2.446
2.464 |
0.9136
0.9059
0.9191
0.9129
0.8538
0.8600 |
6.207
6.207
5.809
5.809
5.729
5.729 |
6.363
6.207
5.846
5.863
5.485
5.377 |
1.0251
1.0000
1.0064
1.0093
0.9574
0.9386 |
|
| 1ratio nbsp;=
response / (µg/mL) |
|
|
|
|
|
4.5 Storage data
Thirty-six samples were
generated by sampling a test atmosphere (one times the target
concentration, 80% RH) at 0.05 L/min for 15 min. Six samples were
analyzed immediately after the generation. Fifteen samples were stored
in a refrigerator (0°C) and the other fifteen were stored in the dark at
ambient temperature (20-25°C). Every few days over a 15-day period,
three samples were selected from each of the two sets and analyzed. The
results are listed Table 4.5. and presented graphically in Figures 4.5.1
and 4.5.2.
Table 4.5
Storage Test
|
storage
time
(days) |
%
recovery
(ambient) |
%
recovery
(refrigerated) |
|
0
0
5
11
12
14
15 |
102.6
94.6
103.6
93.9
80.9
85.6
72.7 |
95.5
102.6
98.6
88.6
85.7
88.4
76.7 |
103.2
101.6
93.9
98.4
83.2
88.2
90.8 |
102.6
94.6
118.0
98.0
83.8
89.6
89.7 |
95.5
102.6
98.9
102.6
85.0
89.6
84.9 |
103.2
101.6
90.4
101.1
102.3
90.3
92.5 |
|
4.6 Precision
(analytical method only)
The precision of the analytical
procedure is 0.034. The precision of the analytical procedure is defined
as the pooled coefficient of variation determined from duplicate
injections of nine analytical standards representing 0.5, 1, and 2 times
the target concentration (Section 4.4).
Table
4.6
Precision of the Analytical Method
(based on the data
of Table 4.4.)
|
| × target
conc. |
0.5× |
1× |
2× |
|
| mean |
0.8204 |
0.8942 |
0.9895 |
| SD |
0.0290 |
0.0293 |
0.0337 |
CV
|
0.0353
|
0.0328
|
0.0341
|
| CV |
= 0.034 |
|
|
|
|
4.7 Precision
(overall procedure)
The precision of the overall procedure is
determined from the storage data. The determination of the standard
error of estimate (SEE) for a regression line plotted through the
graphed storage data allows the inclusion of storage time as one of the
factors affecting overall precision. The SEE is similar to the standard
deviation except it is a measure of dispersion of data about a
regression line instead of about a mean. It is determined with the
following equation:
| SEE = |
[ |
S (Yobs - Yest)2
n-k |
] |
1/2
|
where
n = total no. of data
points
k = 2 for linear regression
k = 3
for quadratic regression
Yobs = observed
% recovery at a given time
Yest =
estimated % recovery form the regression line at the same given
time An additional ±5% for pump
error is added to the SEE by the addition of variances. The precision at
the 95% confidence level is obtained by multiplying the SEE (with pump
error included) by 1.96 (the z-statistic from the standard normal
distribution at the 95% confidence level). The 95% confidence intervals
are drawn about their respective regression lines in the storage graphs
as shown in Figure
4.5.2. The data for Figure 4.5.2. was used to determine the SEE of
±7.51% and the precision of the overall procedure at the 95% confidence
level of 14.7%.
4.8 Reproducibility data
Six
samples, collected from a controlled test atmosphere [80% RH, 20-25°C,
87.7 kPa (658 mmHg)] at the target concentration, were given to a
chemist unassociated with this evaluation. The samples were stored for 1
day at about 0°C before being analyzed. The results are presented in
Table 4.8. No sample result had a percent deviation greater than the
precision of the overall procedure, which was ±14.7%.
Table
4.8
Reproducibility Data
|
| sample no. |
µg found |
µg expected |
% found |
% deviation |
|
1
2
3
4
5
6 |
12.41
12.07
12.04
11.30
11.81
12.22 |
11.24
11.10
11.27
11.32
11.43
11.17 |
110.4
108.7
106.8
99.8
103.3
109.4 |
+10.4
+8.7
+6.8
-0.2
+3.3
+9.4 |
|
4.9 Sampler
capacity
Sampler capacity was tested by sampling a test
atmosphere of 33.4 mg/m3 acetic anhydride at ambient
temperature and 80% relative humidity. Two samplers, each containing
only the front filter, were placed in series and the back sampler was
replaced with a new one every 20 minutes to monitor the downstream air
concentration. The sampling rate was 0.0507 L/min. The data are
presented in Figure
4.9. The 5% breakthrough point, defined as the point where the
downstream analyte concentration reaches 5% of the upstream
concentration, was 180 min.
Table
4.9
Breakthrough Data at 1.7 × Target Concentration
|
time1
(min) |
breakthrough
(%) |
time1
(min) |
breakthrough
(%) |
|
10
30
50
70
90
110
130
150 |
1.0
3.1
1.0
0.6
1.4
1.1
0.8
2.1 |
170
190
210
230
250
270
290
|
4.6
6.3
8.0
10.4
13.3
15.2
18.7
|
|
1midpoint
of each sampling period
4.10 Extraction efficiency and
stability of extracted samples
4.10.1 Extraction efficiency
The extraction efficiency for AcPP was determined by
liquid-spiking 1-2PP-impregnated GFF's with acetic anhydride at the
target concentration (15.75 µg). These samples were stored at ambient
temperature overnight and then extracted and analyzed. The average
extraction efficiency was 97.7%.
Table 4.10.1
Extraction Efficiency
|
| sample no. |
µg spiked |
µg recovered |
% recovered |
|
1
2
3
4
5
6
 |
15.75
15.75
15.75
15.75
15.75
15.75
|
15.42
15.42
15.59
14.61
15.46
15.79
|
97.9
97.9
99.0
92.8
98.2
100.3
97.7 |
|
4.10.2
Stability of extracted samples
The stability of extracted
samples was investigated by reanalyzing the extracted samples with
fresh standards about 24 h after the original analysis. The samples
had been recapped and stored at room temperature. The average of the
reanalyzed samples relative to the average of the original analysis
was 99.8%.
Table 4.10.2
Stability of Extracted Samples
|
original
result
(%) |
reanalyzed
result (%) |
reanlalyzed
relative
to original (%) |
|
97.9
97.9
99.0
92.8
98.2
100.3 |
97.3
100.5
100.0
91.4
100.6
94.9 |
99.4
102.6
101.0
98.5
102.4
94.6 |
| 4.11
Chromatograms
A chromatogram at the detection limit of the
analytical procedure is shown in Figure
4.1 and a chromatogram at the target concentration is shown in Figure
3.5.1.
4.12 Synthesis of AcPP
4.12.1 Reagents
1-(2-Pyridyl)piperazine, 98%, from Aldrich
Acetic
anhydride, reagent grade, from Aldrich
Toluene, from American
Burdick and Jackson
Isooctane, Optima, from Fisher Scientific
Anhydrous sodium carbonate, reagent, from Mallinckrodt
Activated charcoal, from SKC
4.12.2 Apparatus
Erlenmeyer flasks
Filtering flask
Fritted-glass
filtering funnel
4.12.3 Procedure
Add a solution
of 1.63 g of 1-2PP in 25 mL of toluene to a solution of 1.02 g acetic
anhydride in 25 mL of toluene. Stir the mixture for 10 min. Add 2 g of
sodium carbonate (to remove the by-product acetic acid) and let stand
at room temperature overnight. Filter the solution and decolorize with
activated charcoal if desired. Evaporate the toluene to about 25 mL.
Slowly add isooctane until the solution turns cloudy. Add a few drops
of toluene to make the solution clear again. Remove from the hot plate
and let stand at room temperature. Collect the resulting crystals by
filtration. Recrystallize from toluene/isooctane; m.p. 89.5-91.5°C;
quantitative yield.
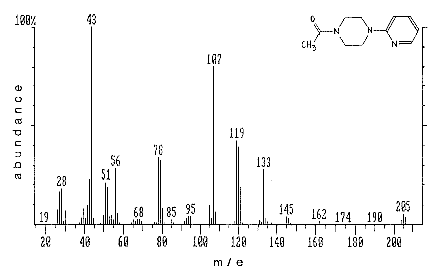
Figure 1.1.4
Molecular structure and mass spectrum of
1-acetyl-4-(2-pyridyl)piperazine. The mass spectrum was obtained
with a Perkin-Elmer ion trap detector.
|
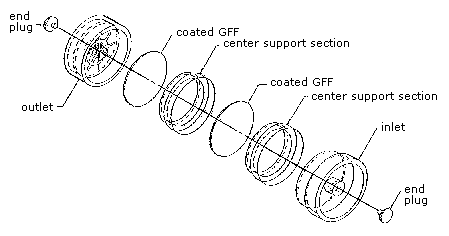
Figure 2.3.1 A
drawing of a sample cassette. |
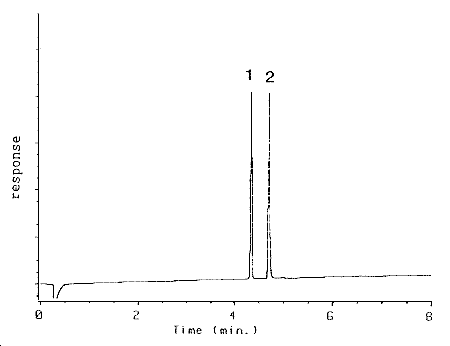
Figure
3.5.1 Chromatogram at target concentration. 1 =
benzalazine, 2 AcPP. |
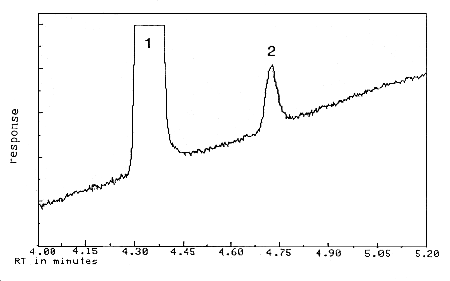
Figure 4.1
Detection limit of the analytical procedure. 1 = benzalazine,
2 = AcPP. |
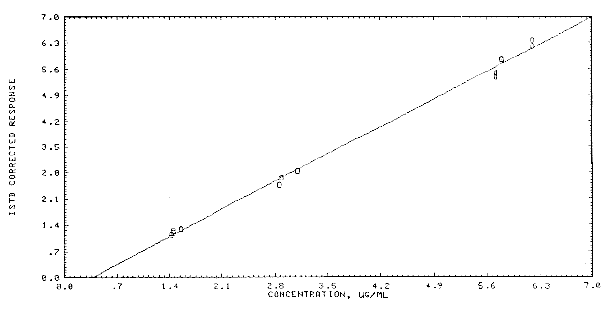
Figure 4.4 Calibration curve for AcPP.
|
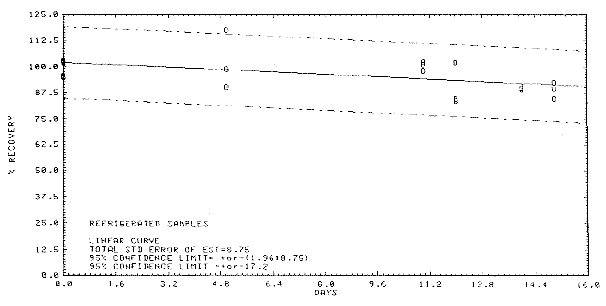
Figure
4.5.1 Storage test at reduced
temperature. |
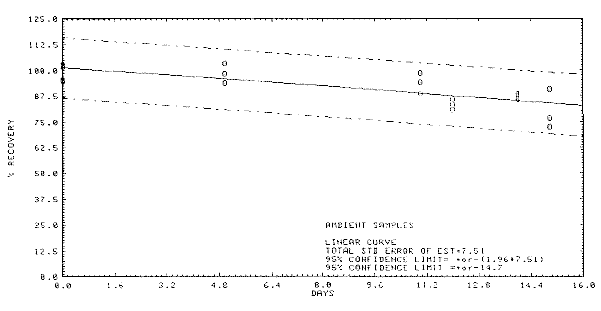
Figure 4.5.2
Storage test at ambient temperature.
|
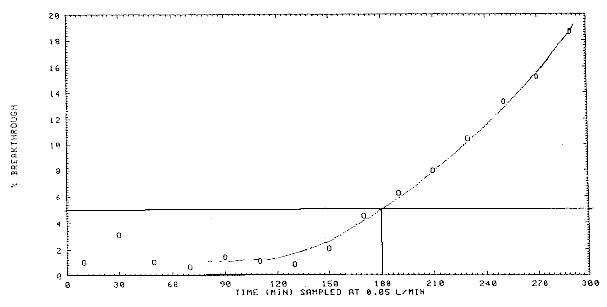
Figure 4.9
Breakthrough curve for acetic anhydride.
|
5. References
5.1 Method No. 3506 in "NIOSH
Manual of Analytical Methods," 3rd edition, 1985 Supplement, U.S.
Department of Health and Human Services, Center for Disease Control,
NIOSH; Cincinnati, OH, 1986.
5.2 Qazi, A.H. and Vincent,
W.J., "Sampling and Analysis of Acetic Anhydride in Air," Am. Ind. Hyg.
Assoc. J., 40(9): 803-808 (1979).
5.3 Langhorst, M.L.,
"Monitoring Airborne Reactive Chemicals by Derivatization and High
Performance Thin Layer Chromatography - Anhydrides, Acid Halides,
Isocyanates," Am. Ind. Hyg. Assoc. J., 46(5): 236-243
(1985).
5.4 Standen, A., Ed., "Kirk-Othmer Encyclopedia of
Chemical Technology," 3rd edition, Volume 1, p. 163. Interscience
Publishers, New York, N.Y., 1984.
5.5 J.T. Baker Material
Safety Data Sheets (MSDS).
5.6 "Air Contaminants -
Permissible Exposure Limits," Code of Federal Regulations, Title 29;
1910.1000, U.S. Department of Labor, OSHA; Washington, D.C., 1989, DOL
(OSHA) Publ. No. OSHA 3112.
5.7 "NIOSH/OSHA Occupational
Health Guidelines for Chemical Hazards," U.S. Department of Health and
Human Services, Government Printing Office, DHHS(NIOSH) Publication No.
81-123.
5.8 Reference 5.4. Volume 1, p.
158.
5.9 Sweet, D.V., Ed., "Registry of Toxic Effects of
Chemical Substances," 1985-86 edition, U.S. Department of Health and
Human Services, Government Printing Office, DHHS(NIOSH) Publication No.
87-114.
5.10 Weast, R.C., Ed., "Handbook of Chemistry and
Physics," 67th edition, Boca Raton, Florida, CRC Press,
1986.
5.11 Author's personal observations.
|

