| Method no.: | 91 |
| Matrix: | Air |
| Target concentration: | TWA 200 ppm (260 mg/m3)
(skin designation) STEL 250 ppm (310 mg/m3) |
| Procedure: | A sample is collected by drawing air through two Anasorb 747 sampling tubes (6-mm i.d. glass tubes, the front tube contains 400 and the back 200 mg of sorbent) which have been connected in series. The samples are desorbed with a carbon disulfide/dimethyl formamide solution and analyzed by gas chromatography with FID detection. |
| Recommended air volume and sampling rate: |
5 L at 0.05 L/min when relative humidity is |
| (Section 2.6) | more than 50% at 25°C (11.5 mg of water per L of
air) 3 L at 0.05 L/min when relative humidity is less than 50% at 25°C (11.5 mg of water per L of air) |
| Reliable quantitation limit: | 142 ppb (186 µg/m3) |
| Standard error of estimate at the target concentration: |
5.24% |
| (Section 4.7.) | |
| Special requirement: | The air sampler must be separated into its two component sampling tubes as soon as possible after sampling. This will prevent post-sampling migration. |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: October 1991 | Chemist: Warren Hendricks |
OSHA Salt Lake Technical Center
Salt Lake City, UT 84165-0200
1. General Discussion
- 1.1. Background
- 1.1.1. History
Previously, OSHA used an in-house modification of a NIOSH method for the sampling and analysis of methyl alcohol. The NIOSH method requires sample collection on silica gel, desorption with water, and analysis by gas chromatography with FID detection. Two samplers are recommended in the NIOSH method; one is a 2-section sampling tube containing 100/50-mg sections of silica gel and the other is a 3-section sampling tube containing 750/150/150-mg sections of silica gel. The 3-section tube is to be used when either high relative humidity or when high levels of methyl alcohol are anticipated. (Ref. 5.1.) The OSHA in-house modification of the NIOSH method consists of sample collection using a 2-section sampling tube containing 520/260-mg sections of silica gel, and desorption with dilute sulfuric acid (Ref. 5.2.).
The results of an ambient temperature storage stability test conducted using silica gel sampling tubes (520/260-mg sections) revealed that extensive migration of methyl alcohol from the 520-mg to the 260-mg sections had occurred upon storage. The average amount found on the 260-mg section was 28% of the total amount on both sections after only 4 days of ambient storage. These results, together with a desire to find a more versatile collection medium, initiated a search for another sorbent. Several sorbents were screened, but only carbon molecular sieves (SKC) and Anasorb 747 (a carbon-based adsorbent produced from pitch) (SKC) had sufficient capacity for methyl alcohol. Both sorbents demonstrated considerably less capacity at low relative humidity than at high relative humidity. These seemingly anomalous capacity results were probably caused by the affinity of methyl alcohol for simultaneously collected water. The capacity of carbon molecular sieves was more affected at low humidity than Anasorb 747, therefore, Anasorb 747 was selected for further evaluation.
Storage tests showed that Anasorb 747, like silica gel, has an ambient storage migration problem. The average amount of methyl alcohol found on the back section of the sampling tube was 16% of the total amount found on both sections after 4 days of ambient storage. Anasorb 747 was selected over silica gel because of the likelihood that other analytes, which may have been collected while sampling for methyl alcohol, can be determined. Methyl alcohol, collected on silica gel, is a "single request" analyte.
The migration problem was circumvented by assembling the air sampler with two separate sampling tubes which can be easily connected before sampling and then separated after sampling. The samples are desorbed with 50/50 carbon disulfide/DMF solution for 1 h. The high percentage of DMF is necessary to put any collected water into solution, thus eliminating the possibility of 2-phase samples.
The recommended air sample volume for methyl alcohol collected on Anasorb 747 must be reduced at lower relative humidities because sampler capacity is reduced at low relative humidity. Fifty-percent relative humidity at 25°C (11.5 mg of water per liter of air) was selected as the point at which to reduce the recommended air volume. The 50% relative humidity point will provide a sufficient margin of safety against sampler saturation. It is anticipated that most samples will be collected at relative humidities greater than 50% at 25°C. Air temperature has a significant effect on the water content of humid air. This effect is addressed in Section 2.6.
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
Occupational exposure to methyl alcohol can occur through ingestion, inhalation, and absorption through the skin or the eyes. Ingestion of the liquid, or exposure to high concentrations of the vapor, can cause blindness and death. Toxic effects include severe acidosis marked by the metabolic production of formaldehyde and formic acid. Symptoms of chronic exposure include reduction of vision acuity, conjunctivitis, headache, giddiness, insomnia, and gastric disturbances. Direct skin contact can cause dermatitis, erythema, and scaling. (Refs. 5.3. and 5.4.)
1.1.3. Workplace exposure
Methyl alcohol is commercially produced by the high pressure catalytic reaction of carbon monoxide with hydrogen, and by the partial oxidation of natural gas hydrocarbons. It can also be produced by the gasification of wood, peat, and lignite. The U.S. production of methyl alcohol was nearly 8 billion pounds in 1990. It is used to manufacture formaldehyde and other chemicals. It is also used as a solvent for nitrocellulose, ethyl cellulose, and various natural and synthetic resins and dyes; as a denaturant for ethyl alcohol; as an antifreeze; as a dehydrator for natural gas; and as a fuel. (Refs. 5.5. and 5.6.)
NIOSH estimated that approximately 175 thousand U.S. workers were potentially exposed to methyl alcohol in 1976 (Ref. 5.7.).
1.1.4. Physical properties (Refs. 5.3. and 5.4.)
| CAS no. | 67-56-1 |
| molecular weight: | 32.04 |
| physical description: | colorless, mobile, highly polar, flammable liquid |
| specific gravity: | 0.7915 at 20°C |
| boiling point: | 64.5°C |
| melting point: | -97.8°C |
| vapor density: | 1.11 (air = 1) |
| vapor pressure: | 12.3 kPa (92 mmHg) at 20°C |
| flash point: | 54°F (12.2°C) (closed cup) |
| chemical formula: | CH3OH |
| autoignition temp: | 878°F (470°C) |
| explosive limits: | 6.7% and 36.5% by volume in air |
| solubility: | miscible with water, ethyl alcohol, ether, and many other organic solvents |
| synonyms: | methanol; wood alcohol; Columbian spirits; carbinol |
The analyte air concentrations listed throughout this method are
based on an air volume of 5 L and a solvent extraction volume of 3.0 mL.
Air concentrations listed in ppm and ppb are referenced to
- 1.2. Limit defining parameters
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 24 pg per injection. This is the amount of methyl alcohol which will produce a peak with a height about 5 times the height of the baseline noise. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 0.93 µg per sample (142 ppb or 186 µg/m3). This is the amount of methyl alcohol spiked on the sampler that, upon analysis, produces a peak similar in size to that of the detection limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 0.93 µg per sample (142 ppb or 186 µg/m3). This is the smallest amount of methyl alcohol which can be quantitated within the requirements of a recovery of at least 75% and a precision (±1.96 SD) of ±25% or better. (Section 4.3.)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Instrument response to the analyte
The instrument response over the concentration range of 0.5 to 2 times the target concentration is linear. (Section 4.4.)
1.2.5. Recovery
The recovery of methyl alcohol from samples used in a 18-day storage test remained above 88% when the samples were stored at ambient temperature (Section 4.5.).
1.2.6. Precision (analytical procedure)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1, and 2 times the target concentration is 0.0048. (Section 4.6.)
1.2.7. Precision (overall procedure)
The precision at the 95% confidence level for the 18-day ambient temperature storage test is ±10.27%. (Section 4.7.) This includes an additional ±5% for pump error.
1.2.8. Reproducibility
Six samples collected from a controlled test atmosphere and a draft copy of this procedure were given to a chemist unassociated with the evaluation. The samples were analyzed after 14 days of storage at about -2°C. No individual sample deviated from its theoretical value by more than the precision reported in Section 1.2.7. (Section 4.8.)
1.3. Advantages
- 1.3.1. This sampling procedure prevents post-sampling migration
of methyl alcohol on the sampler.
1.3.2. This analytical procedure should permit the determination of other analytes which may have been collected while sampling for methyl alcohol.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. A sample is collected by use of a personal sampling pump
that can be calibrated to within ±5% of the recommended 0.05 L/min
flow rate with the sampler in line.
2.1.2. A sample is collected using a 400-mg and a 200-mg Anasorb 747 sampling tube that have been connected in series with a 1-in. length of 1/4-in. i.d. silicone tubing. The glass sampling tubes are 6-mm i.d. × 8-mm o.d. containing either 400 mg or 200 mg of Anasorb 747. The sorbent is held in place with a glass wool plug at the front and a foam plug at the end of the sorbent bed. The sampling tubes are commercially available from SKC as catalog no. 226-82.
2.2. Reagents
No sampling reagents are required.
2.3. Technique
- 2.3.1. Break off both ends of each 400-mg and 200-mg sampling
tube. The holes in the broken ends of the tubes should be
approximately 1/2 the i.d. of the sampling tube. Connect the outlet
end of the 400-mg tube to the inlet end of the 200-mg sampling tube
with a 1-in. length of 1/4-in. i.d. silicone rubber tubing. The
inlet end of a sampling tube is the end with the glass wool plug.
Insure that the connection is secure and that the broken ends of the
sampling tubes just touch each other. Be careful not to cut the
silicone tubing with the sharp ends of the sampling tubes.
2.3.2. Attach the sampler to the sampling pump with flexible, plastic tubing so that the inlet end of the 400-mg tube of the sampler is exposed directly to the atmosphere.
2.3.3. Attach the sampler vertically in the worker's breathing zone in such a manner that it does not impede work performance or safety.
2.3.4. Remove the sampler after sampling for the appropriate time. Separate the two sampling tubes and seal the tube ends with plastic end caps. Silicone tubing is susceptible to cuts by the sharp ends of the sampling tubes and should be discarded after one use. Be certain to properly identify the sampling tubes. Wrap the samples end-to-end with an official OSHA seal (Form 21).
2.3.5. Submit at least one blank with each set of samples. The blank should be handled the same as the other samples except that no air is drawn through it.
2.3.6. List any potential interferences on the sample data sheet.
2.4. Sampler capacity
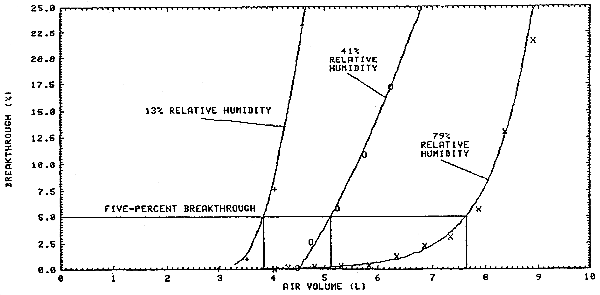
Sampler capacity was evaluated by sampling controlled test atmospheres with a front sampling tube (400-mg tube) and several back sampling tubes (200-mg tubes). The back sampling tubes were used to monitor the effluent from the front sampling tube and were changed at measured time intervals. Five-percent breakthrough from the front tube was used as evidence that sampling capacity had been exceeded and was defined as the point at which the methyl alcohol concentration in the effluent from the front tube was 5% of the concentration of the test atmosphere.
Three sampler capacity experiments were performed at different relative humidities. The water content of the sampled air at the studied relative humidities (RH) and temperatures is presented in Table 2.4. The average methyl alcohol concentration of the test atmospheres was 420 ppm and sampling was performed at 0.05 L/min. (Section 4.9.)
Sampler Capacity at Different Relative Humidities
|
| |||
| RH | temperature | amt. water | 5% breakthrough |
| (%) | (°C) | (mg/L of air) | (L) |
|
| |||
| 79 | 22 | 15.3 | 7.6 |
| 41 | 25 | 9.4 | 5.1 |
| 13 | 22 | 2.5 | 3.8 |
|
| |||
The data in Table 2.4. clearly shows that the water content of the sampled air has a substantial effect on sampler capacity.
2.5. Desorption efficiency
- 2.5.1. The average desorption efficiency of methyl alcohol from
Anasorb 747 over the range of from 0.5 to 2 times the target
concentration was 100%. (Section 4.10.)
2.5.2. Desorbed samples remain stable for at least 16 h. (Section 4.10.)
2.5.3. Desorption efficiencies should be confirmed periodically because differences may occur due to variations in Anasorb 747 lots, desorption solvent, and operator technique.
2.6. Recommended air volume and sampling rate
- 2.6.1. Sample 5 L of air at 0.05 L/min for TWA samples when the
relative humidity is above 50% at 25°C (11.5 mg of water per L of
air).
Relative humidity is defined as the percentage ratio of water vapor present in air (at a specified temperature) relative to the quantity of water vapor that would saturate that air at the same temperature. Air temperature has a significant effect on the water content of air. Air at 50% relative humidity and 25°C contains 11.5 mg/L of water, however, air at the same relative humidity and 10°C contains only 4.7 mg/L. Therefore, both air temperature and relative humidity must be considered when determining the water content of air.
Use the data (Ref. 5.8.) in Table 2.6.1. and the air temperature
in degrees Centigrade
Water Content of Saturated Humid Air
|
| |||||||
| temp. | water | temp. | water | temp. | water | temp. | water |
| (°C) | (mg/L) | (°C) | (mg/L) | (°C) | (mg/L) | (°C) | (mg/L) |
|
| |||||||
| -20 | 1.07 | 15 | 12.83 | 23 | 20.58 | 31 | 32.07 |
| -10 | 2.36 | 16 | 13.63 | 24 | 21.78 | 32 | 33.83 |
| 0 | 4.85 | 17 | 14.48 | 25 | 23.05 | 33 | 35.68 |
| 5 | 6.80 | 18 | 15.37 | 26 | 24.38 | 34 | 37.61 |
| 10 | 9.40 | 19 | 16.31 | 27 | 25.78 | 35 | 39.63 |
| 12 | 10.66 | 20 | 17.30 | 28 | 27.24 | 36 | 41.75 |
| 13 | 11.35 | 21 | 18.34 | 29 | 28.78 | 37 | 43.96 |
| 14 | 12.07 | 22 | 19.43 | 30 | 30.38 | 38 | 46.26 |
|
| |||||||
Example no. 1: What air volume should be collected when air at the sampling site is at 27°C and 84% relative humidity? Answer: Water content = 0.84(25.78) = 21.66 mg/L; 21.66 mg/L is greater than 11.5 mg/L; collect a 5-L air sample under these conditions.
2.6.2. Reduce the sample volume to 3 L when the relative humidity falls below 50% at 25°C (11.5 mg of water per L of air).
Example no. 2: What air volume should be collected when air at the sampling site is at 14°C and 67% relative humidity? Answer: Water content = 0.67(12.07) = 8.09 mg/L; 8.09 mg/L is less than 11.5 mg/L; collect a 3-L air sample under these conditions.
2.6.3. Collect 15-min samples at 0.05 L/min for STEL samples.
2.6.4. When short-term samples are required, the reliable quantitation limit becomes larger. For example, the reliable quantitation limit is 0.95 ppm (1.24 mg/m3) for methyl alcohol when 0.75 L of air is collected.
2.7. Interferences (sampling)
- 2.7.1. It is unknown if any compound(s) will severely interfere
with the collection of methyl alcohol on Anasorb 747. In general,
the presence of other solvents will reduce the capacity of Anasorb
747 to collect methyl alcohol. Low relative humidity can reduce the
capacity of Anasorb 747 to collect methyl alcohol.
2.7.2. A sampling interference study was performed by sampling test atmospheres containing methyl alcohol, toluene, and butyl cellosolve. This mixture was selected because a solvent mixture containing 69% methyl alcohol, 26% toluene, and 5% butyl cellosolve has been used in the workplace. The concentrations of the test atmospheres were 408-mg/m3 methyl alcohol, 154-mg/m3 toluene, 29.6-mg/m3 butyl cellosolve. Both humid (76% relative humidity at 26°C) and dry (23% relative humidity at 24°C) test atmospheres were generated. The air sample volumes collected from the humid atmosphere were 4, 5, and 6 L and those for the dry atmosphere were 2, 3, and 4 L. No excessive breakthrough was observed in any of the samples and the average recoveries were: methyl alcohol, 94%; toluene, 88%; and butyl cellosolve, 91%. Toluene and butyl cello-solve recoveries were not corrected for desorption efficiency.
2.7.3. Report any potential interference which is used in the sampling area when submitting samples to the laboratory.
2.8. Safety precautions (sampling)
- 2.8.1. Attach the sampling equipment to the worker in such a
manner that it will not interfere with work performance or safety.
2.8.2. Wear eye protection when breaking the ends of the Anasorb 747 sampling tubes. Take suitable precautions against cuts when connecting the sampling tubes.
2.8.3. Follow all safety practices that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A GC equipped with a flame ionization detector. A
Hewlett-Packard 5890 GC equipped with a 7673A Automatic Sampler was
used in this evaluation.
3.1.2. A GC column capable of resolving methyl alcohol from the desorbing solvent and potential interferences. A 60-m × 0.32-mm i.d., 1-m df Stabilwax fused silica capillary column (Restek catalog no. 10657) was used in this evaluation.
3.1.3. An electronic integrator or some other suitable means to measure detector response. A Waters 860 Networking Computer System was used in this evaluation.
3.1.4. Vials, glass, 4-mL and 2-mL, with PTFE-lined caps.
3.1.5. Volumetric flasks, pipets and syringes for preparing standards, making dilutions and performing injections.
3.1.6. Pipets, disposable, Pasteur-type.
3.2. Reagents
- 3.2.1. Methyl alcohol, reagent grade or better. b&j Brand,
High Purity Solvent, lot no. AW-106 was used in this evaluation.
3.2.2. Desorbing solution, 50/50 carbon disulfide and dimethyl
formamide (DMF), reagent grade or better. Fisher Scientific carbon
disulfide (lot no. 743869) and Burdick and Jackson DMF (lot no.
A-1649) was used in this evaluation.
3.2.3. Water, deionized grade or better. Laboratory deionized water was used in this evaluation.
3.3. Standard preparation
- 3.3.1. Prepare stock standards by diluting methyl alcohol with
water. Prepare analytical standards by diluting the stock standards
with desorbing solution. For example, a stock standard containing
77.4 mg/mL was prepared by diluting 1.0 mL of methyl alcohol to 10.0
mL with water. An analytical standard was prepared from this stock
standard by diluting 17.0 µL of the stock with 3.0 mL of desorbing
solution. The concentration of the analytical standard was 1315.8
µg/sample and it was approximately equivalent to the amount that
would be collected in a 5-L air sample at 200 ppm.
3.3.2. Prepare a sufficient number of standards to generate a calibration curve. Analytical standard concentrations must bracket sample concentrations.
3.4. Sample preparation
- 3.4.1. Transfer the contents of each sampling tube to separate
4-mL glass vials. Discard the glass wool and foam plugs.
3.4.2. Add 3.0 mL of desorbing solution to each vial using the same dispenser used to prepare standards. Seal the vials with PTFE-lined caps.
3.4.3. Desorb the samples for 1 h. Shake the vials vigorously several times during the desorption time.
3.4.4. Transfer an aliquot of the desorbed sample to an autosampler vial if necessary.
3.5. Analysis
- 3.5.1. GC conditions
| column: | Restek 1-m df Stabilwax, 60-m × 0.32-mm i.d. |
| temperatures: | 200°C (injector) 250°C (detector) 40°C (column, initial temp) |
| temperature program: | hold initial temp 1 min, increase temp at 10°C/min to 240°C, hold temp 2 min |
| gas flow rates: | 2.0 mL/min (column, hydrogen) 3.2 mL/min (septum purge, hydrogen) 43 mL/min (FID, hydrogen) 33 mL/min (FID make-up, nitrogen) 415 mL/min (FID, air) |
| injection volume: | 1-µL (13:1 split) |
| retention times: | 8.4 min (methyl alcohol) 16.2 min ( |
3.5.2. A chromatogram at the target concentration is shown in Figure 3.5.2.
3.5.3. An internal standard (ISTD) calibration method should be used. Prepare a calibration curve by plotting the ISTD corrected detector response for each standard solution against its respective actual concentration in micrograms of methyl alcohol per sample. Determine the best-fit line through the data points by curve fitting. Sample results must be bracketed by standard concentrations.
3.6. Interferences (analytical)
- 3.6.1. Any compound which produces an FID response and has a
similar retention time as methyl alcohol or the internal standard is
a potential interference. Potential interferences which were
reported when the samples were submitted for analysis should be
considered before desorbing the samples.
3.6.2. Retention time on a single column is not proof of chemical identity. Confirmation of suspected identity should be performed by GC/mass spectrometry when necessary.
3.7. Calculations
- 3.7.1. The concentration, micrograms of methyl alcohol per
sample, is determined from the calibration curve. If methyl alcohol
is found on the back sampling tube, it is added to the amount found
on the front tube. Blank corrections should be performed before
adding the results together.
3.7.2. The methyl alcohol air concentration can be expressed using the following equation:
| mg/m3 = | (A)
(B) (C) |
| where | A | = | micrograms per sample (from Section 3.7.1.) |
| B | = | liters of air sampled | |
| C | = | desorption efficiency (decimal form) |
3.7.3. The following equation can be used to convert methyl alcohol results in mg/m3 to ppm at 25°C and 101.3 kPa (760 mmHg):
| ppm = | (mg/m3) (24.46)
32.04 |
| where | mg/m3 | = | result from Section 3.7.2. |
| 24.46 | = | molar volume at 101.3 kPa (760 mmHg) and 25°C | |
| 32.04 | = | molecular weight of methyl alcohol |
3.8. Safety precautions (analytical)
- 3.8.1. Avoid skin contact and inhalation of all chemicals.
3.8.2. Restrict the use of all chemicals to a fume hood.
3.8.3. Wear safety glasses and a lab coat in all lab areas.
4. Backup Data
- 4.1. Detection limit of the analytical procedure
The injection size recommended in the analytical procedure (1-L, 13:1 split) was used to determine the detection limit of the analytical procedure. The detection limit of the analytical procedure was 24 pg on-column per injection. This was the amount of methyl alcohol that gave a peak with a height about 5 times the height of the baseline noise. This detection limit was determined by the analysis of a standard containing 0.31 µg/mL of methyl alcohol. Figure 4.1. is a chromatogram of the detection limit of the analytical procedure.
4.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 0.93 µg per sample (142 ppb or 186 µg/m3). The injection size recommended in the analytical procedure (1-µL, 13:1 split) was used in the determination of the detection limit of the overall procedure. Six vials, each containing 400 mg of Anasorb 747 sorbent were each liquid spiked with 0.93 µg of methyl alcohol. The samples were desorbed after 2 days of ambient storage in a hood.
Detection Limit of the Overall Procedure
|
| ||
| sample | theoretical amount | amount recovered |
| number | (µg) | (µg) |
|
| ||
| 1 | 0.93 | 1.08 |
| 2 | 0.93 | 1.10 |
| 3 | 0.93 | 1.08 |
| 4 | 0.93 | 0.99 |
| 5 | 0.93 | 0.99 |
| 6 | 0.93 | 0.98 |
|
| ||
4.3. Reliable quantitation limit data
The reliable quantitation limit is also 0.93 µg per sample (142 ppb or 186 µg/m3). The injection size recommended in the analytical procedure (1 µL, 13:1 split) was used in the determination of the reliable quantitation limit. Because the recovery of methyl alcohol from spiked samples (Section 4.2.) was greater than 75% and also because the precision (±1.96 SD) was less than ±25%, the detection limit of the overall procedure and reliable quantitation limit are the same.
Reliable Quantitation Limit
(based on samples and data of Table 4.2.)
|
| |||
| percent | statistics | ||
| recovered | |||
|
| |||
| 116.1 | |||
| 118.3 | = | 111.4 | |
| 116.1 | SD | = | 6.0 |
| 106.4 | Precision | = | (1.96)(±6.0) |
| 106.4 | = | ±11.8% | |
| 105.4 | |||
|
| |||
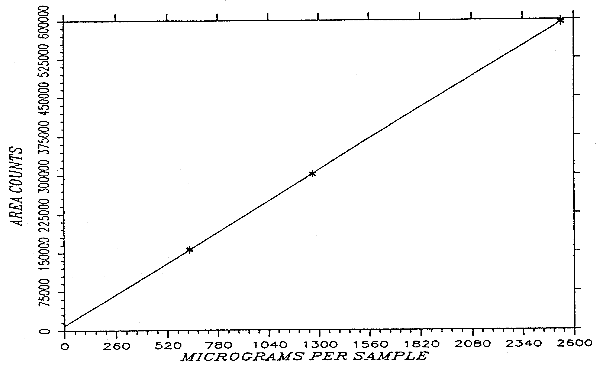
4.4. Instrument response to methyl alcohol
The instrument response to methyl alcohol over the range of 0.5 to 2 times the target concentration is linear with a slope of 240 area counts per microgram per sample. The response to methyl alcohol was determined by multiple injections of standards. The data in Table 4.4. is presented graphically in Figure 4.4.
Instrument Response to Methyl Alcohol
|
| |||
| × target concn | 0.5× | 1× | 2× |
| µg/sample | 633.6 | 1267.2 | 2534.4 |
|
| |||
| area counts | 157668 | 300536 | 599269 |
| 157318 | 302499 | 596338 | |
| 156057 | 301148 | 595879 | |
| 156773 | 300970 | 593236 | |
| 155840 | 301961 | 594505 | |
| 154659 | 300773 | 593236 | |
| |
156386 | 301314 | 595410 |
|
| |||
4.5. Storage test
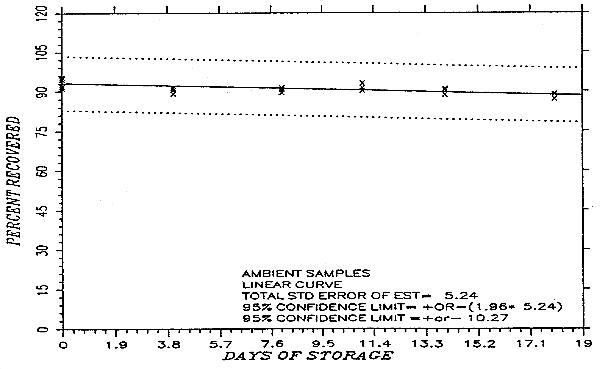
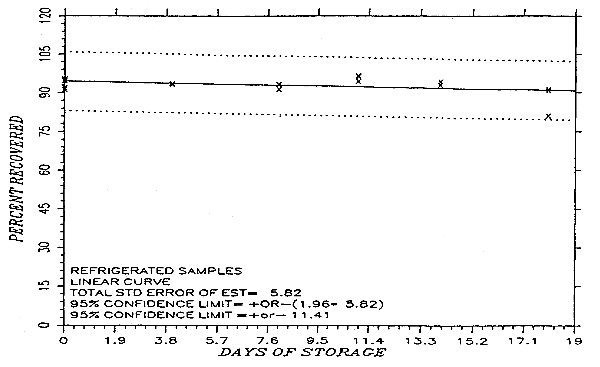
Thirty-six samples were collected by sampling a test atmosphere containing 526 mg/m3 methyl alcohol for about 50 min at 0.05 L/min. Storage samples are usually collected by sampling a test atmosphere at the target concentration for the recommended time at the recommended sampling rate. However, the concentration of the test atmosphere was doubled and the sampling time was halved for the generation of storage samples used in this study. This was done so that ambient and refrigerated samples could be collected on the same day. The relative humidity of the atmosphere was 60% at 27°C. Eighteen of the samples were stored in a refrigerator at -2°C and the other eighteen were stored in the dark at ambient temperature (about 24°C). Every few days, three samples were selected from each of the two storage sets and analyzed. The storage data are also presented graphically in Figures 4.5.1. and 4.5.2.
Storage Test
|
| |||||||
| storage | % recovery | % recovery | |||||
| time (days) | (ambient) | (refrigerator) | |||||
|
| |||||||
| 0 | 94.7 | 91.2 | 94.6 | 94.8 | 91.9 | 95.4 | |
| 0 | 94.8 | 91.9 | 95.4 | 94.7 | 91.2 | 94.6 | |
| 4 | 91.3 | 89.2 | 90.5 | 93.3 | 93.8 | 93.8 | |
| 8 | 89.5 | 91.3 | 90.6 | 93.4 | 91.3 | 93.1 | |
| 11 | 93.1 | 90.3 | 91.2 | 96.7 | 94.4 | 96.3 | |
| 14 | 90.5 | 88.7 | 91.0 | 94.4 | 93.1 | 92.9 | |
| 18 | 88.7 | 89.0 | 87.0 | 81.0 | 90.8 | 91.4 | |
|
| |||||||
4.6. Precision (analytical method)
The precision of the analytical procedure is defined as the pooled coefficient of variation determined from replicate injections of methyl alcohol standards at 0.5, 1, and 2 times the target concentration.
Precision of the Analytical Method
(based on the data of Table 4.4.)
|
| |||
| × target concn | 0.5× | 1× | 2× |
| µg/sample | 633.6 | 1267.2 | 2534.4 |
|
| |||
| SD1 | 1100.07 | 757.19 | 2291.04 |
| CV | 0.0070 | 0.0025 | 0.0038 |
|
| |||
| 1 standard deviation is in area counts | |||
4.7. Precision (overall procedure)
The precision of the overall procedure is determined from the storage data. The determination of the standard error of estimate (SEE) for a regression line plotted through the graphed storage data allows the inclusion of storage time as one of the factors affecting overall precision. The SEE is similar to the standard deviation except it is a measure of dispersion of data about a regression line instead of about a mean. It is determined with the following equation:
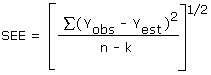
| where | n | = | total no. of data points |
| k | = | 2 for linear regression | |
| k | = | 3 for quadratic regression | |
| Yobs | = | observed % recovery at a given time | |
| Yest | = | estimated % recovery from the regression line at the same given time |
An additional ±5% for pump error is added to the SEE by the addition of variances. The precision at the 95% confidence level is obtained by multiplying the SEE (with sampling error included) by 1.96 (the z-statistic from the standard normal distribution at the 95% confidence level). The 95% confidence intervals are drawn about their respective regression lines in the storage graphs as shown in Figures 4.5.1. and 4.5.2. The data for Figure 4.5.1. was used to determine the SEE of ±5.24% for methyl alcohol.
4.8. Reproducibility
Six samples, collected from a controlled test atmosphere were assigned to a chemist unassociated with this study. The concentration of the test atmosphere was 206 ppm methyl alcohol and the relative humidity was 59% at 26°C. The samples were analyzed after 14 days of storage at about -2°C. One sample was lost during analysis. The sample results are corrected for desorption efficiency. No sample result had a percent deviation greater than the precision of the overall procedure which was ±10.27%.
Reproducibility Data
|
| |||
| µg collected | µg recovered | % recovered | % deviation |
|
| |||
| 1135.18 | 1136.43 | 100.1 | +0.1 |
| 1210.85 | 1190.55 | 98.3 | -1.7 |
| 1148.69 | 1116.73 | 97.2 | -2.8 |
| 1486.54 | 1429.91 | 96.2 | -3.8 |
| 1483.84 | 1389.21 | 93.3 | -6.7 |
|
| |||
4.9. Sampler capacity
Sampler capacity was evaluated by sampling controlled test atmospheres with a front sampling tube (400-mg tube) and several back sampling tubes (200-mg tubes). The back sampling tubes were used to monitor the effluent from the front sampling tube. A back sampling tube was connected to a front tube and then sampling was commenced. Sampling was discontinued, after a measured time interval, while the existing back tube was replaced with a fresh back tube and then sampling was resumed. The methyl alcohol concentration in the effluent was determined with the air volume sampled during each interval. Percent breakthrough was calculated by dividing the concentration in the effluent by the concentration of the test atmosphere and then multiplying the result by 100.
Three sampler capacity experiments were performed at different relative humidities. The average concentration of the test atmospheres was 420 ppm and sampling was performed at 0.05 L/min.
The air volumes in Table 4.9. are cumulative and were calculated using the midpoint of each measured time interval. The results of the capacity tests are also presented graphically in Figure 4.9.
Sampler Capacity Data
|
| |||||
| 79% RH, 22°C | 41% RH, 25°C | 13% RH, 22°C | |||
| air vol (L) | bt (%) | air vol (L) | bt (%) | air vol (L) | bt (%) |
|
| |||||
| 4.05 | 0.0 | 4.49 | 0.1 | 2.97 | 0.0 |
| 4.30 | 0.2 | 4.74 | 2.6 | 3.51 | 1.0 |
| 4.82 | 0.3 | 5.25 | 5.8 | 4.05 | 7.7 |
| 5.32 | 0.4 | 5.75 | 10.9 | 4.59 | 23.2 |
| 5.84 | 0.4 | 6.25 | 17.2 | ||
| 6.34 | 1.3 | 6.76 | 24.9 | ||
| 6.86 | 2.3 | ||||
| 7.36 | 3.1 | ||||
| 7.88 | 5.7 | ||||
| 8.38 | 13.0 | ||||
| 8.92 | 21.7 | ||||
|
| |||||
| RH = relative humidity | bt = breakthrough | ||||
4.10. Desorption efficiency and stability of extracted samples
The desorption efficiency for methyl alcohol was determined by liquid spiking 400-mg portions of Anasorb 747 contained in separate glass vials with a solution containing methyl alcohol in water. These samples were stored at room temperature overnight and then desorbed and analyzed. The average desorption efficiency (DE) was 100.3%.
Desorption Efficiency Data
|
| |||
| × target concn | 0.5× | 1× | 2× |
| µg/sample | 633.6 | 1267.2 | 2534.4 |
|
| |||
| DE, | 101.2 | 98.8 | 99.8 |
| % | 103.3 | 99.1 | 98.1 |
| 101.8 | 99.2 | 100.4 | |
| 103.7 | 99.3 | 98.8 | |
| 102.5 | 100.3 | 98.5 | |
| 105.0 | 97.4 | 99.1 | |
| 102.9 | 99.0 | 99.1 | |
|
| |||
About 16 h after the initial analysis, both the original 1× target concentration sample aliquots and also fresh aliquots from the 1× desorption vial containing the sorbent were reanalyzed using freshly prepared standards. The average of the reanalyzed samples was 99.3% of the original analysis.
Desorption Stability Data
|
| |||
| original | original | fresh | |
| analysis | aliquot | aliquot | |
|
| |||
| DE, | 98.8 | 95.8 | 98.1 |
| % | 99.1 | 97.5 | 98.3 |
| 99.2 | 96.6 | 99.5 | |
| 99.3 | 98.5 | 99.4 | |
| 100.3 | 99.4 | 101.0 | |
| 97.4 | 97.9 | 97.7 | |
| 99.0 | 97.6 | 99.0 | |
|
| |||
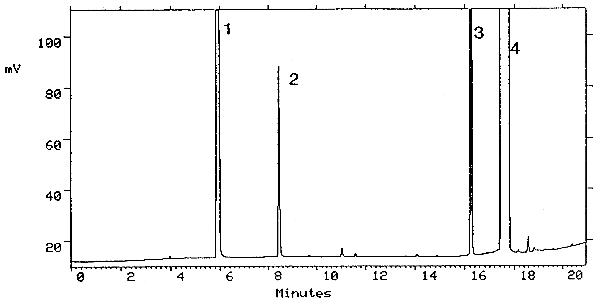
Figure 3.5.2. methyl alcohol chromatogram at the target
concentration. Peak identification was as follows: 1, carbon disulfide;
2, methyl alcohol; 2,
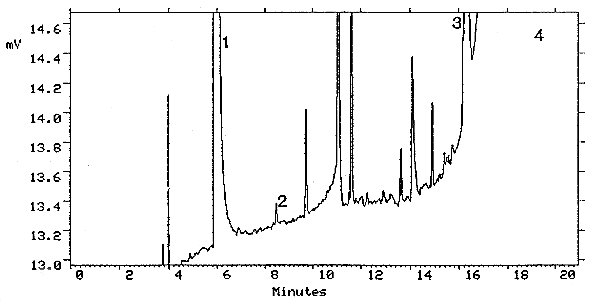
Figure 4.1. Detection limit of the analytical procedure for methyl
alcohol. Peak identification was as follows: 1, carbon disulfide; 2,
methyl alcohol; 3,
5. References
- 5.1. "NIOSH Manual of Analytical Methods", 3rd ed. Vol. 2; U.S.
Department of Health and Human Services, Public Health Service,
Centers for Disease Control, National Institute for Occupational
Safety and Health, Division of Physical Sciences and Engineering;
Cincinnati, OH, 1984, Method 2000, DHHS (NIOSH).
5.2. OSHA Computerized Information System Database, SLCAL Chemical Sampling Information, Methyl Alcohol, REV 900508.
5.3. "NIOSH/OSHA Occupational Health Guidelines for Chemical Hazards", U.S. Dept. of Health and Human Services, Public Health Service, Center for Disease Control, NIOSH and U.S. Dept. of Labor, OSHA: U.S. Government Printing Office Washington, DC, Jan 1981, Methyl Alcohol, DHHS (NIOSH) Publ. No. 81-123.
5.4. "Documentation of the Threshold Limit Values and Biological Indices", 5th ed.; American Conference of Governmental Industrial Hygienists (ACGIH): Cincinnati, ISBN: 0-036712-68-6, 1986; p 372.
5.5. "Hawley's Condensed Chemical Dictionary", 11th ed., Sax, N.l. and Lewis, R.J. Eds., Van Nostrand Reinhold, New York, 1987, p. 667.
5.6. Chemical and Engineering News, Vol. 69, No. 25, June 24, 1991, p. 31.
5.7. "Criteria for a Recommended Standard...Occupational Exposure to Methyl Alcohol", U.S. Department of Health, Education, and Welfare, PHS/CDC/NIOSH, March, 1976, HEW Publication No. (NIOSH) 76-148.
5.8. "CRC Handbook of Chemistry and Physics", 67th ed., Weast, R.C. Ed., CRC Press, Boca Raton, FL, 1986-7, p. E-37.