![]()
| Method no.: | 95 |
| Matrix: | Air |
| Target concentration: | 60 µg/m3 |
| Procedure: | Samples are collected open-face by drawing air through a
|
| Recommended air volume and sampling rate: |
480 L at 2.0 L/min |
| Reliable quantitation limit: | 1.39 µg/m3 |
| Standard error of estimate at the target concentration: (Section 4.7.) |
6.8% |
| Special requirement: | Samples should be stored in a refrigerator when not in transit. |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: August 1992 | Chemist: Yihlin Chan |
OSHA Salt Lake Technical Center
Salt Lake City, UT 84165-0200
1. General Discussion
- 1.1. Background
- 1.1.1. History
Airborne ethylene thiourea has been determined by collection on
PVC or
In this method, airborne ethylene thiourea is collected on glass fiber filters and analyzed by HPLC. Compared to colorimetirc analysis, HPLC is specific, speedy, and sensitive. Glass fiber filters were selected because they are better suited for HPLC analysis than the membrane filters.
Although ethylene thiourea has been shown to be an animal carcinogen and NIOSH recommends that it be handled as if it were a human carcinogen and teratogen (Ref. 5.8.), there is no PEL or TLV. The target concentration used in this evaluation was arbitrarily set at 60 µg/m3, approximately 40 times the reliable quantitation limit.
1.1.2. Toxic effects (This section is for information only and
should not be taken as the basis of OSHA policy.) (Refs.
Ethylene thiourea is an antithyroid substance and animal carcinogen. In groups of rats fed 125 or 625 ppm for up to 90 days, marked increases in serum thyroid stimulating hormone were found. Ethylene thiourea produced a high incidence of follicular carcinoma of the thyroid in rats after oral administration. Ethylene thiourea is believed to induce thyroid tumors through the suppression of thyroxine syntheses, leading to hyperplasia of the thyroid gland. Ethylene thiourea was a potent teratogen in rats at doses that produced no maternal toxicity or fetal deaths. Ethylene thiourea is a skin, eye, and mucous membrane irritant to laboratory animals.
Human toxicity data is limited. One reported a clinical examination and thyroid function tests carried out over a period of 3 years on 13 exposed workers showed one subgroup (the mixers) to have significantly lower levels of total thyroxine than other workers (Ref. 5.9.). In another incidence study, approximately 2,000 workers were identified as having worked at some time with ethylene thiourea in one of several rubber manufacturing companies and in one firm producing ethylene thiourea. No case of thyroid cancer was reported in this group (Ref. 5.10.). The IARC has determined that there is sufficient evidence for carcinogenicity of ethylene thiourea to animals but inadequate evidence for humans (Ref. 5.11.).
1.1.3. Workplace exposure
Workers in the rubber industry face potential occupational
exposure to ethylene thiourea because it is used extensively as an
accelerator in the curing of polychloroprene (Neoprene) and other
elastomers. NIOSH estimated that approximately 3500 workers in the
rubber industry have potential occupational exposure to ethylene
thiourea. This estimate was based on the NIOSH National Occupational
Hazard Survey conducted between 1972 and 1974 (Ref.
5.8.). No recent data was found. Although ethylene thiourea has
been proposed for a variety of uses and although numerous patents
have been issued, no evidence was found that it is being used
commercially for other than
1.1.4. Physical properties and other descriptive information
(Refs.
| CAS no.: | 96-45-7 |
| synonyms: | 2-imidazolidinethione;
|
| structural formula: | |
| molecular wt: | 102.17 |
| melting point: | 203-204°C |
| odor: | faint amine odor |
| appearance: | white crystalline solid |
| solubility: | solubility in 100 mL water: 2 g at 30°C, 9 g at 60°C, 44 g at 90°C. Moderately soluble in methanol, ethanol, ethylene glycol, and pyridine; insoluble in acetone, ether, chloroform, benzene, ligroin. |
The analyte air concentrations throughout this method are based on the recommended sampling and analytical parameters.
1.2. Limit defining parameters
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 2.1 ng per
injection
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 0.666 µg per sample (1.39 µg/m3). This is the amount of analyte spiked on the sampling device that, upon analysis, produces a peak similar in size to that of the detection limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 0.666 µg per sample (1.39 µg/m3). This is the smallest amount of analyte which can be quantitated within the requirements of a recovery of at least 75% and a precision (±1.96 SD) of ±25% or better. (Section 4.3.)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
1.2.4. Instrument response to the analyte
The instrument response over concentration ranges representing 0.5 to 2 times the target concentration is linear. (Section 4.4.)
1.2.5. Recovery
The recovery of ethylene thiourea from samples used in a 15-day ambient storage test remained above 88.3%. (Section 4.5.)
1.2.6. Precision (analytical procedure)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1 and 2 times the target concentration is 0.016. (Section 4.6.)
1.2.7. Precision (overall procedure)
The precision at the 95% confidence level for the 15-day ambient temperature storage test is ±13.3%. (Section 4.7.) This includes an additional ±5% for sampling error.
1.2.8. Reproducibility
Six samples, collected from a test atmosphere of ethylene thiourea, and a draft copy of this procedure were given to a chemist unassociated with this evaluation. The samples were analyzed after 11 days of storage at 5°C. No individual sample result deviated from its theoretical value by more than the precision reported in Section 1.2.7. (Section 4.8.)
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. A personal sampling pump that can be calibrated within
±5% of the recommended flow rate with the sampling device in line.
2.1.2. A four-piece polystyrene cassette containing two glass fiber filters assembled as shown.
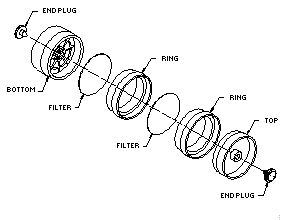
2.2. Reagents
No reagents are required for sampling.
2.3. Technique
- 2.3.1. Prepare the sampler for open-face sampling by removing
the top piece and the end plug from the bottom piece. Attach the
sampler to the sampling pump with a piece of flexible tubing and
place it in the worker's breathing zone with the open face of the
cassette facing down.
2.3.2. Replace the top piece and the two end plugs after
sampling. Seal each sample with an official Form
2.3.3. Submit at least one blank with each set of samples. Handle the blank the same as the other samples except draw no air through it.
2.3.4. List any potential interferences on the sample data sheet.
2.4. Sampler capacity
Sampling capacity exceeds 276 µg, or 720 L at 6.4 times the target concentration and at a sampling rate of 2.0 L/min. This loading is equivalent to 2300 L at 2 times the target concentration. (Section 4.9.)
2.5. Extraction efficiency (Section 4.10.)
- 2.5.1. The average extraction efficiency of ethylene thiourea
from glass fiber filters is 99.9%.
2.5.2. Extracted samples remain stable for at least 24 h.
2.6. Recommended air volume and sampling rate
- 2.6.1. For TWA samples the recommended air volume is 480 L
collected at 2.0 L/min.
2.6.2. For short term samples the recommended air volume is 30 L
collected at 2.0 L/min
2.6.3. When short term samples are required, the reliable quantitation limit becomes larger. For example, the reliable quantitation limit is 22 µg/m3 when 30 L of air is collected.
2.7. Safety precautions (sampling)
Attach the sampling equipment to the worker in such a manner that it will not interfere with work performance or safety. Follow all safety practices applicable to the work area.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. An HPLC equipped with a UV detector. A BAS 200 HPLC
(Bioanalytical Systems) and Waters WISP auto-sampler were used in
this evaluation.
3.1.2. An HPLC column capable of separating ethylene thiourea from any interferences. An Alltech C18 column (4.6 × 250 mm) was used in this evaluation.
3.1.3. An electronic integrator or other suitable means of measuring detector response. A Waters 860 Networking Computer System was used in this evaluation.
3.1.4. Sample vials, 4-mL glass, with polytetrafluoroethylene-lined caps.
3.1.5. A mechanical shaker.
3.2. Reagents
- 3.2.1. Ethylene thiourea. Ethylene thiourea, 98%, was obtained
from Aldrich and recrystallized from methanol.
3.2.2. Water, HPLC grade. Water was obtained from a Millipore Milli-Q water purification system.
3.2.3. Methanol, HPLC grade. Methanol, Optima grade, was obtained from Fisher Scientific.
3.3. Standard preparation
- 3.3.1. Prepare stock standards by weighing 10-20 mg of ethylene
thiourea in
3.3.2. Prepare a sufficient number of analytical standards to generate a calibration curve. Analytical standard concentrations must bracket sample concentrations.
3.4. Sample preparation
- 3.4.1. Transfer the two filters to separate 4-mL glass vials.
3.4.2. Add 3.0 mL of water to each vial.
3.4.3. Cap the vials and shake them on a mechanical shaker for 30 min.
3.5. Analysis
- 3.5.1. HPLC conditions
| column: | Alltech C18 | 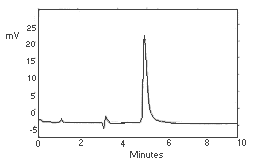 Figure 3.5. Chromatogram of ethylene thiourea at target concentration. |
| eluent: | methanol/water 10:90 (v/v) | |
| flow rate: | 1.0 mL/min | |
| injection vol: | 10 µL | |
| retention time: | 5.3 min | |
| UV detector: | 234 nm |
3.5.2. Construct a calibration curve using an external standard method by plotting micrograms per milliliter versus detector response of standards.
3.6. Interferences (analytical)
- 3.6.1. Any compound that absorbs at 234 nm and has a similar
retention time as the analyte is a potential interference.
Generally, chromatographic conditions can be altered to separate an
interference from the analyte.
3.6.2. Retention time on a single column is not considered proof of chemical identity. Additional means of identification include: GC/MS, analysis using an alternate HPLC column, detection at another wavelength (peak ratioing), and analysis by GC/FID.
3.7. Calculations
The analyte amount for a sample is obtained from the calibration curve in terms of micrograms per milliliter uncorrected for extraction efficiency. The back filter is analyzed primarily to determine if there was any breakthrough from the front filter during sampling. If a significant amount of analyte is found on the back filter (e.g., greater than 25% of the amount found on the front filter), this fact should be reported with sample results. If any analyte is found on the back filter, it is added to the amount on the front filter. The analyte amount is then corrected by subtracting the total amount found in the blank. The air concentration is obtained by using the following equation.
|
|
3.8. Safety precautions (analytical)
- 3.8.1. Restrict the use of all chemicals to a fume hood.
3.8.2. Avoid skin contact and inhalation of all chemicals.
3.8.3. Wear safety glasses, gloves and a lab coat at all times while working with chemicals.
4. Backup Data
The injection size recommended in the analytical procedure
|
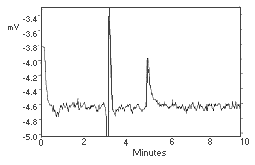 Figure 4.1. Chromatogram of the analytical detection limit. |
4.2. Detection limit of the overall procedure
| The detection limit of the overall procedure was determined by analyzing filters liquid spiked with 0.666 µg of ethylene thiourea. This amount corresponds to an air concentration of 1.39 µg/m3. The injection size listed in the analytical procedure (10 µL) was used in the determination of the detection limit of the overall procedure. |
| |||||||||||||||
| 4.3. Reliable quantitation limit
The reliable quantitation limit was determined by analyzing filters liquid spiked with 0.666 µg of ethylene thiourea. This amount corresponds to an air concentration of 1.39 µg/m3. Because the recovery of the analyte from the spiked samples was greater than 75% with a precision of ±25% or better, the detection limit of the overall procedure and reliable quantitation limit are the same. |
| |||||||||||||||
4.4. Instrument response to the analyte
The instrument response to ethylene thiourea over the range of 0.5 to 2 times the target concentration was determined from multiple injections of analytical standards. The response is linear with a slope of 2.43 × 105 area counts per microgram per milliliter.
|
| |||
| × target concn µg/mL |
0.5× 4.76 |
1.0× 9.52 |
2.0× 19.04 |
|
| |||
| area counts | 1036261 1010263 1051344 1023608 1022236 1024264 |
2207258 2193614 2262707 2307770 2214339 2164339 |
4517505 4512732 4497015 4493679 4492322 4502761 |
|
| |||
| mean | 1027996 | 2225005 | 4502669 |
|
| |||
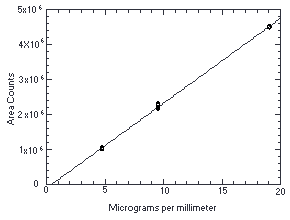
Figure 4.4.
Calibration curve for ethylene thiourea.
4.5. Storage data
Storage samples were pre-pared from a test atmosphere of ethylene
thiourea aerosol generated by pumping an isopropanol solution of
ethylene thiourea (0.4 mg/mL) at a rate of 0.4 mL/min through a TSI
Model 3076 atomizer (TSI Incorporated, St. Paul, MN), where it was
dispersed with an air stream of 3.5 L/min. The aerosol passed through
an electrostatic charge neutralizer and was mixed with a dilution air
stream of 97 L/min (25°C, 80% RH). The diluted aerosol flowed into a
chamber fitted with 18 sampling ports.
|
| |||||||
| days of storage |
% recovery (ambient) |
% recovery (refrigerated) | |||||
|
| |||||||
| 0 0 2 5 8 12 15 |
104.0 100.4 96.3 99.6 96.2 87.6 89.7 |
94.3 100.7 101.2 97.9 93.1 94.1 77.9 |
96.1 104.5 92.0 97.7 94.7 100.7 88.1 |
104.0 100.4 95.4 100.4 103.2 97.3 100.2 |
94.3 100.7 99.0 106.5 97.5 105.9 99.0 |
96.1 104.5 104.1 96.6 99.5 98.4 99.8 | |
|
| |||||||
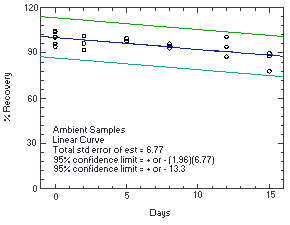 Figure 4.5.1. Storage test at ambient temperature. |
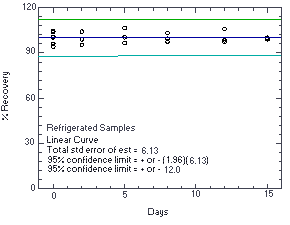 Figure 4.5.2. Storage test at reduced temperature (5°C). |
| 4.6. Precision (analytical method)
The precision of the analytical procedure is defined as the pooled coefficient of variation determined from replicate injections of analytical standards representing 0.5, 1, and 2 times the target concentration. The coefficients of variation are calculated from the data in Table 4.4. The pooled coefficient of variation is 0.016. |
| ||||||||||||||||||||
4.7. Precision (overall procedure)
The precision of the overall procedure is determined from the storage data. The determination of the standard error of estimate (SEE) for a regression line plotted through the graphed storage data allows the inclusion of storage time as one of the factors affecting overall precision. The SEE is similar to the standard deviation, except it is a measure of dispersion of data about a regression line instead of about a mean. It is determined with the following equation:
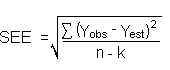
| where | |
| n = k = k = |
total no. of data points 2 for linear regression 3 for quadratic regression |
| Yobs = | observed % recovery at a given time |
| Yest = | estimated % recovery from the regression line at the same given time |
An additional 5% for pump error is added to the SEE by the addition
of variances. The precision at the 95% confidence level is obtained by
multiplying the SEE (with pump error included) by 1.96 (the
4.8. Reproducibility data
Six samples were collected from a test atmosphere of ethylene thiourea aerosol at 25°C and 80% RH. A draft copy of this method and the samples were submitted to a chemist unassociated with this evaluation for analysis. All of the sample results were within the range of ±13.3%, the precision of the overall procedure.
|
| |||
| µg expected |
µg recovered |
percent recovered |
percent deviation |
|
| |||
| 80.8 80.6 78.3 79.1 79.9 80.3 |
87.8 82.7 77.8 88.0 86.2 84.5 |
108.7 102.6 99.4 111.3 107.9 105.2 |
+8.7 +2.6 -0.6 +11.3 +7.9 +5.2 |
|
| |||
4.9. Sampler capacity
The sampler capacity was assessed by sampling from a dynamically generated test atmosphere of ethylene thiourea (25°C, 80% RH) at 2.0 L/min and for various sampling times analyzing the back filter. At 2 times the target concentration and a sampling time of 6 h, no ethylene thiourea was detected on the back filter. At 0.384 mg/m3 (6.4 times the target concentration) and 6 h, the average amount collected on the front filter was 278 µg and on the back filter, 2.4 µg. This sample loading is equivalent to 2300 L at 2 times the target concentration. Separately, two glass fiber filters were each spiked with 63 µg of ethylene thiourea and humid air (80% RH) was pulled through them at 2 L/min for 226 min. The recovery was 99.5%.
4.10. Extraction efficiency and stability of extracted samples
- 4.10.1. Extraction efficiency
To determine the extraction efficiency, six glass fiber filters were liquid spiked with ethylene thiourea at the target concentration. These samples were stored overnight at ambient temperature and then extracted with water and analyzed. The average recovery was 99.9%.
|
| |||
| sample no. |
µg spiked |
µg recovered |
% recovery |
|
| |||
| 1 2 3 4 5 6 |
28.81 28.81 28.81 28.81 28.81 28.81 |
28.64 28.38 28.81 29.93 28.88 28.03 |
99.4 98.5 100.0 103.9 100.2 97.3 |
|
| |||
| mean | 99.9 | ||
|
| |||
4.10.2. Stability of extracted samples
The stability of extracted samples was ascertained by reanalyzing the above samples 24 h later with fresh standards. The samples were stored at room temperature and were not recapped. The average recovery was 100.3%.
|
| ||
| initial recovery (%) | recovery after 24 h (%) | percent change |
|
| ||
| 99.4 98.5 100.0 103.9 100.2 97.3 |
99.6 99.8 98.9 104.5 99.5 99.3 |
+0.2 +1.3 -1.1 +0.6 -0.7 +2.0 |
|
| ||
5. References
- 5.1. Palassis, J., "Sampling and Analytical
Determination of Airborne Tetramethyl and Ethylene Thiourea", Am.
Ind. Hyg. Assoc. J., 1980, 41(2),
5.2. "NIOSH Manual of Analytical Methods", 3rd
ed.; U.S. Department of Health and Human Services, Center for Disease
Control, National Institute of Occupational Safety and Health;
Cincinnati, OH, 1984, Method 5011, DHHS (NIOSH) Publ. No.
5.3. Prince, J.L., "Analysis of Ethylenethio-urea
in Urine by High Performance Liquid Chromatography",J. Agric. Food
Chem., 1985, 33(1),
5.4. Kurttio, P., T. Vartiainen, and K.
Savolainen, "A High Performance Liquid Chromatographic Method for the
Determination of Ethylenethiourea in Urine and on Filters", Anal.
Chim. Acta, 1988, 212(1/2),
5.5. Onley, J.H., and G. Yip, "Determination of
Ethylene Thiourea Residues in Foods, Using Thin ZLayer and Gas
Chromatography", J. Assoc. Off. Anal. Chem., 1971,
54,
5.6. Farrington, D.S., and R.G. Hopkins,
"Determination of Ethylenethiourea in Ethylenebisdithiocarbamate
Fungicides: Comparison of
5.7. Lehotay, J., E. Brandsteterova, and D.
Oktavec,
5.8. "Current Intelligence Bulletin 22: Ethylene
Thiourea", U.S. Department of Health, Education, and Welfare, Center
for Disease Control, National Institute of Occupational Safety and
Health, DHEW (NIOSH) Publication No.
5.9. Hathaway, G.J., et al., Ed., "Proctor and Hughes' Chemical Hazards of the Workplace", 3rd ed., Van Nostrand Reinhold, New York, 1991.
5.10. "IARC Monographs on the Evaluations of
Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42",
International Agency for Research on Cancer, Lyon, 1987; Supplement 7,
pp
5.11. "IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Man: Some Antithyroid and Related
Substances, Nitrofurans and Industrial Chemicals", International
Agency for Research on Cancer, Lyon, 1974; Vol. 7, pp
5.12. Budavari, S., Ed., "Merck Index", 11th ed., Merck & Co., Rahway, NJ, 1989
5.13. Sweet, D.V., Ed., "Registry of Toxic
Effects of Chemical Substances",