![]()
| Method number: | 106 |
| Matrix: | Air |
| Target concentrations: | 1 ppm (6.9 mg/m3) and 75 ppm (515 mg/m3) |
| OSHA PEL: | None |
| ACGIH TLV: | None |
| Procedure: | Samples are collected by drawing a known volume of air through standard size (6-mm o.d., 140/70) Anasorb 747 tubes. Samples are desorbed with toluene and analyzed by GC using a flame ionization detector (FID). |
| Recommended air volume and sampling rate: |
3 L at 0.05 L/min |
| Reliable quantitation limit: | 33.1 ppb (228 µg/m3) |
| Standard error of estimate at the target concentrations: |
5.3% at 1 ppm 5.6% at 75 ppm |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: April 1995 | Chemist: Donald Burright |
Organic Methods Evaluation Branch
OSHA Salt Lake
Technical Center
Salt Lake City, UT 84165-0200
1. General Discussion
- 1.1 Background
- 1.1.1 History
This method is an extension of the work that was done earlier to produce OSHA Method 103. (Ref. 5.1) Desflurane was identified as a new anesthetic gas undergoing testing for FDA approval during the earlier work. FDA approved desflurane for use in the general population in September 1992. Desflurane was not included in Method 103 because it required different analytical conditions. Initial studies were conducted using both Anasorb CMS and Anasorb 747 as adsorbents for collection of desflurane to parallel the method used for isoflurane, halothane and enflurane. Anasorb 747 was selected as the adsorbent for collection because a higher desorption efficiency was obtained. The desorbing solvent was changed from carbon disulfide to toluene. This reduced the loss of desflurane from the sample which was caused by the heat generated when carbon disulfide was added to the adsorbent. The evaluation was performed at two target concentrations, 1 and 75 ppm, to parallel the earlier work. There is no OSHA PEL or ACGIH TWA for desflurane, but NIOSH has a recommended exposure limit (REL) for halogenated anesthetic gases of 2 ppm as a 60-min ceiling value (Refs. 5.2 and 5.3). Because OSHA sometimes sets the TWA concentration at about one-half of the ceiling value, the REL is the basis for the lower target concentration. The higher target concentration was chosen because of the close structural similarity between desflurane and enflurane, which has an ACGIH TLV of 75 ppm.
1.1.2 Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
Acute exposure to desflurane may cause irritation and redness to the eyes, and dryness and irritation to the skin. Overexposure by inhalation can lead to headaches, dizziness, drowsiness, unconsciousness or death. Irritation of the mouth and throat can occur with an acute exposure by inhalation. Acute exposure by ingestion may lead to unconsciousness or death. (Ref. 5.4)
1.1.3 Workplace exposure
Desflurane is a new organic anesthetic gas and may be found in operating rooms, teaching hospitals, dental offices, and veterinary hospitals. The number of people potentially exposed is not known but will rise as desflurane is accepted into general use.
1.1.4 Physical properties and other descriptive information (Ref. 5.4)
| CAS number: molecular weight: boiling point, °C: color: specific gravity: molecular formula: vapor pressure, kPa (mmHg): odor: flash point: synonyms: |
57041-67-5 168.04 22.8 clear 1.47 @ 15°C C3H2OF6 89.2 (669.2) @ 20°C mild, ethereal >93°C (CC) Suprane™; 1,2,2,2-tetrafluoroethyl difluoromethyl ether; |
| structural formula: | 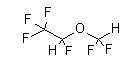 |
The analyte air concentrations throughout this method are based on the recommended sampling and analytical parameters. Air concentrations listed in ppm and ppb are referenced to 25°C and 101.3 kPa (760 mmHg).
- 1.2 Limit defining parameters
- 1.2.1 Detection limit of the analytical procedure
The detection limit of the analytical procedure is 13.6 pg. This is the amount of analyte that will give a response that is significantly different from the background response of a reagent blank. (Sections 4.1 and 4.2)
1.2.2 Detection limit of the overall procedure
The detection limit of the overall procedure is 0.205 µg per sample (9.93 ppb or 68.4 µg/m3). This is the amount of analyte spiked on the sampler that will give a response that is significantly different from the background response of a sampler blank. (Sections 4.1 and 4.3)
1.2.3 Reliable quantitation limit
The reliable quantitation limit is 0.683 µg per sample (33.1 ppb or 228 µg/m3). This is the amount of analyte spiked on a sampler that will give a signal that is considered the lower limit for precise quantitative measurements. (Section 4.4)
1.2.4 Precision (analytical procedure)
The precisions of the analytical procedure, measured as the pooled relative standard deviation over a concentration range equivalent to the range of 0.5 to 2 times the target concentration are 0.58% and 0.46% for the lower and higher target concentrations, respectively. (Section 4.5)
1.2.5 Precision (overall procedure)
The precisions of the overall procedure at the 95% confidence level for the ambient temperature 18- and 16-day storage tests (at the target concentration) are 10.4% and 11.0% for the lower and higher target concentrations, respectively. This includes an additional 5% for sampling error. (Section 4.6)
1.2.6 Recovery
The recoveries of desflurane from samples used in the 18- and 16-day storage tests remained above 99.7% and 100.2% for the lower and higher target concentrations, respectively when the samples were stored at 22°C. (Section 4.7)
1.2.7 Reproducibility
Twelve samples spiked by liquid injection were submitted for analysis by one of the OSHA Salt Lake Technical Center's service branch laboratories. The samples were analyzed according to the instructions in a draft copy of this procedure after 10 days of storage at 4°C. No individual sample result deviated from its theoretical value by more than the precision reported in Section 1.2.5. (Section 4.8)
2. Sampling Procedure
- 2.1 Apparatus
- 2.1.1 Samples are collected using a personal sampling pump
calibrated, with the sampling device attached, within ±5% at the
recommended flow rate.
2.1.2 Samples are collected with 7-cm × 4-mm i.d. × 6-mm o.d. glass sampling tubes packed with two sections of Anasorb 747 (140/70 mg). The sections are held in place with a glass wool plug and two urethane foam plugs. For this evaluation, commercially prepared sampling tubes were purchased from SKC, Inc. (catalog no. 226-81A).
2.2 Reagents
None required.
2.3 Technique
- 2.3.1 Only properly trained personnel can sample in an operating
room or dental office, this is necessary to be in compliance with
OSHA's Exposure Control Plan for bloodbourne pathogens. (Ref. 5.5)
2.3.2 Break off the ends of the sampling tube immediately before sampling. All tubes should be from the same lot.
2.3.3 Attach the sampling tube to the sampling pump with flexible, non-crimpable tubing. It is desirable to utilize sampling tube holders which have a protective cover to shield the employee from the sharp, jagged end of the sampling tube. Position the tube so that the sampled air first passes through the larger section.
2.3.4 Sampled air should not pass through any hose or tubing before entering the sampling tube.
2.3.5 Attach the sampler vertically with the larger section pointing downward in the worker's breathing zone to avoid channeling. Position the sampler so it does not impede work performance or safety.
2.3.6 Remove the sampling tube and seal it with plastic end caps immediately after sampling for the appropriate time.
2.3.7 In order t prevent occupational exposure to SLTC personnel, sampling tubes that may become contaminated with blood or other potentially infectious materials are to be examined prior to shipping and decontaminated (e.g., wiped off with bleach or other disinfectant) as necessary. Contaminated items are not to placed or stored in areas where food is kept, and decontamination should be accomplished as soon as possible following the inspection where contamination occurred. Decontamination is not to take place in any area where food or drink is consumed. (Ref. 5.5)
2.3.8 Wrap each sample end-to-end with a Form OSHA-21 seal.
2.3.9 Submit at least one blank sample with each set of samples. Handle the blank sampling tube in the same manner as the other samples, except draw no air through it.
2.3.10 Record sample air volumes (in liters) for each sample, along with any potential interferences.
2.3.11 Ship any bulk sample(s) in a container separate from the air samples.
2.3.12 Submit the samples to the laboratory for analysis as soon as possible after sampling. If delay is unavoidable, store the samples at reduced temperature.
2.4 Sampler capacity
Sampler capacity is determined by measuring how much air can be sampled before the analyte breaks through the sampler, i.e., the sampler capacity is exceeded. Breakthrough is considered to occur when the effluent from the sampler contains a concentration of analyte that is 5% of the upstream concentration (5% breakthrough). Testing for breakthrough was performed by using an FID to monitor the effluent from sampling tubes containing only the 140-mg section of Anasorb 747. Dynamically generated test atmospheres, which were about two times the higher target concentration, were used for the capacity tests. The samples were collected at 0.05 L/min and the relative humidity was about 80% at 25°C. The 5% breakthrough air volume was calculated from the data of duplicate determinations and is 3.83 L. (Section 4.9)
2.5 Desorption efficiency
- 2.5.1 The average desorption efficiencies for desflurane from
Anasorb 747 over the range of 0.5 to 2.0 times the target
concentrations were 101.1% and 102.9% for the lower and higher
target concentration respectively. (Section 4.10)
2.5.2 The desorption efficiencies at 0.05, 0.1 and 0.2 times the target concentrations (TC) were found to be very high and are listed below. (Section 4.10)
| Table 2.5.2 Desorption Efficiencies at 0.05 to 0.2 times TC, % | |||
| TC | 0.05×TC | 0.1×TC | 0.2×TC |
|
| |||
| low high |
98.0 100.0 |
103.2 100.2 |
100.2 101.0 |
|
| |||
2.5.3 Desorbed samples remain stable for at least 20 and 33 h for the lower and higher target concentration respectively.
2.6 Recommended air volume and sampling rate
- 2.6.1 For long-term samples, collect 3 L at 0.05 L/min.
2.6.2 For short-term samples, collect 0.75 L at 0.05 L/min.
2.6.3 When short-term samples are collected, the air concentration equivalent to the reliable quantitation limit becomes larger. For example, the reliable quantitation limit is 133 ppb (911 µg/m3) when 0.75 L is sampled.
2.7 Interferences (sampling)
- 2.7.1 There are no known compounds that will severely interfere
with the collection of desflurane on Anasorb 747. In general, the
presence of other contaminant vapors in the air will reduce the
capacity of Anasorb 747 to collect desflurane.
2.7.2 Suspected interferences should be reported to the laboratory with submitted samples.
2.8 Safety precautions (sampling)
- 2.8.1 The sampling equipment should be attached to the worker in
such a manner that it will not interfere with work performance or
safety.
2.8.2 All safety practices that apply to the work area being sampled should be followed.
2.8.3 Protective eyewear should be worn when breaking the ends of the glass sampling tubes.
3. Analytical Procedure
- 3.1 Apparatus
- 3.1.1 Gas chromatograph equipped with an FID. For this
evaluation, a Hewlett-Packard 5890A Gas Chromatograph equipped with
a 7673A Automatic Sampler was used. A Forma Scientific Model 2006
refrigerated circulator was used to cool the sample tray of the HP
7673A to 10°C.
3.1.2 A GC column capable of separating the analyte of interest
from the desorption solvent, internal standard and any
interferences. A
3.1.3 An electronic integrator or some other suitable means of measuring peak areas. A Waters 860 Networking Computer System was used in this evaluation.
3.1.4 Two-milliliter vials with poly(tetrafluoroethylene)-lined caps.
3.1.5 A dispenser capable of delivering 1.0 mL of desorbing
solvent to prepare standards and samples. If a dispenser is not
available, a
3.2 Reagents
- 3.2.1 Desflurane, USP. The desflurane used in this evaluation
was manufactured by Anaquest (Liberty Corner, NJ).
3.2.2 Toluene, chromatographic grade or better. The toluene used in this evaluation was Optima Grade and was purchased from Fisher Scientific (Fair Lawn, NJ).
3.2.3 Desorption solvent. The desorption solvent was toluene and the benzene contaminant was used as the internal standard.
3.2.4 GC grade nitrogen, air, and hydrogen.
3.3 Standard preparation
- 3.3.1 Prepare two concentrated stock standards of desflurane in
toluene. Prepare working analytical standards by injecting
microliter amounts of concentrated stock standards into 2-mL vials
containing 1 mL of desorption solvent delivered from the same
dispenser used to desorb samples. For example, to prepare a target
level standard of desflurane, inject 20 µL of a stock
solution containing 77.37 mg/mL of desflurane in toluene into 1 mL
of desorption solvent.
3.3.2 Bracket sample concentrations with working standard concentrations. If samples fall outside of the concentration range of prepared standards, prepare and analyze additional standards to ascertain the linearity of response.
3.4 Sample preparation
- 3.4.1 Remove the plastic end caps from the sample tube and
carefully transfer each section of the adsorbent to separate 2-mL
vials. Discard the glass tube, urethane foam plugs and glass wool
plug.
3.4.2 Add 1.0 mL of desorption solvent to each vial using the same dispenser as used for preparation of standards.
3.4.3 Immediately seal the vials with poly(tetrafluoroethylene)-lined caps.
3.4.4 Shake the vials vigorously several times during the next 30 min.
3.5 Analysis
- 3.5.1 Analytical conditions
| GC conditions | |
| zone | |
| temperatures: | 60°C (column), hold 4 min, ramp at 5°C/min to
90°C, hold 0 min, ramp at 20°C/min to 150°C, hold 5
min 250°C (injector) 300°C (detector) |
| run time: | 18 min |
| column gas flow: | 2.7 mL/min (hydrogen) |
| septum purge: | 1.9 mL/min (hydrogen) |
| injection size: | 1.0 µL (15.5 : 1 split) |
| column: | 60-m × 0.32-mm i.d. capillary SPB-1 (4.0-µm df) |
| retention times: | 3.05 min (desflurane) 10.7 min (benzene) |
| FID conditions | |
| hydrogen flow: | 38 mL/min |
| air flow: | 450 mL/min |
| makeup flow: | 30 mL/min (nitrogen) |
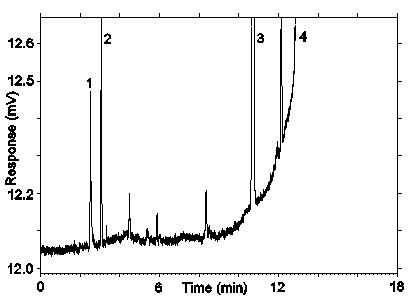
Figure 3.5.1.1. Chromatogram obtained at the low TC with the recommended conditions. Peak identification: (1) air peak, (2) desflurane, (3) benzene, (4) toluene.
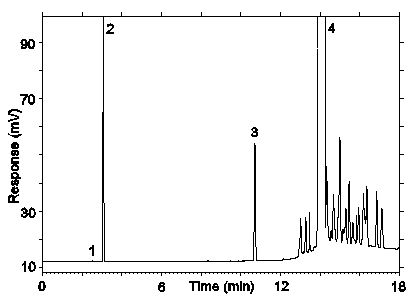
Figure 3.5.1.2. Chromatogram obtained at the hight TC with the recommended conditions. Peak identification: (1) air peak, (2) desflurane, (3) benzene, (4) toluene.
3.5.2 An internal standard (ISTD) calibration method is used. A
calibration curve can be constructed by plotting
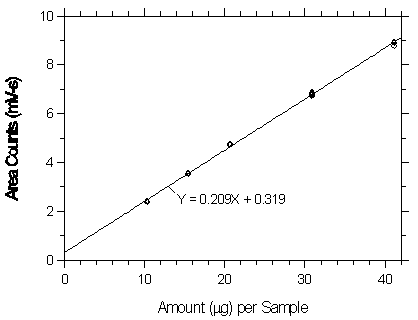
Figure 3.5.2.1. Calibration curve at the low TC made from data of Table 4.5.1.
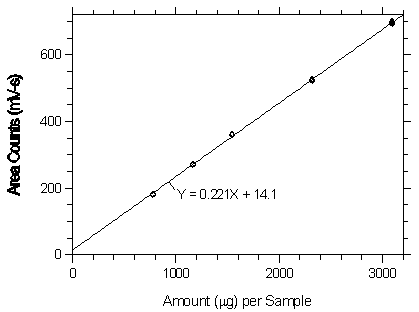
Figure 3.5.2.2. Calibration curve at the high TC made from data of Table 4.5.2.
3.6 Interferences (analytical)
- 3.6.1 Any compound that produces an FID response and has a
similar retention time as the analyte or internal standard is a
potential interference. If any potential interferences were
reported, they should be considered before the samples are desorbed.
Generally, chromatographic conditions can be altered to separate an
interference from the analyte.
3.6.2 When necessary, the identity or purity of an analyte peak may be confirmed with additional analytical data (Section 4.11).
3.7 Calculations
The amount of desflurane per sample is obtained from the appropriate calibration curve in terms of micrograms per sample, uncorrected for desorption efficiency. The back (70-mg) section is analyzed primarily to determine if there was any breakthrough from the front (140-mg) section during sampling. If a significant amount of analyte is found on the back section (e.g., greater than 25% of the amount found on the front section), this fact should be reported with the sample results. If any analyte is found on the back section, it is added to the amount on the front section. This amount is then corrected by subtracting the total amount (if any) found on the blank. The air concentration is calculated using the following formulae.
| mg/m3 = | liters of air sampled × desorption efficiency |
| ppm = | molecular weight of analyte |
where 24.46 is the molar volume at 25°C and 101.3 kPa (760
mmHg)
168.04 = molecular weight
of desflurane
3.8 Safety precautions (analytical)
- 3.8.1 Adhere to the rules set down in your Chemical Hygiene
Plan.
3.8.2 Avoid skin contact and inhalation of all chemicals.
3.8.3 Wear safety glasses, gloves and a lab coat while in the laboratory areas and working with chemicals.
4. Backup Data
- 4.1 Determination of detection limits
Detection limits, in general, are defined as the amount (or concentration) of analyte that gives a response (YDL) that is significantly different (three standard deviations (SDBR)) from the background response (YBR).
The measurement of YBR and SDBR in chromatographic methods is typically inconvenient and difficult because YBR is usually extremely low. Estimates of these parameters can be made with data obtained from the analysis of a series of analytical standards or samples whose responses are in the vicinity of the background response. The regression curve obtained for a plot of instrument response versus concentration of analyte will usually be linear. Assuming SDBR and the precision of the data about curve are similar, the standard error of estimate (SEE) for the regression curve can be substituted for SDBR in the above equation. The following calculations derive a formula for DL:
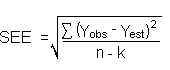
| Yobs | = observed response |
| Yest | = estimated response from regression curve |
| n | = total number of data points |
| k | = 2 for linear regression curve |
At point YDL on the regression curve
YDL = A(DL) + YBR A = analytical sensitivity (slope)
therefore
| DL = | (YDL - YBR)
A |
Substituting 3(SEE) + YBR for YDLgives
| DL = | 3(SEE)
A |
4.2 Detection limit of the analytical procedure (DLAP)
The DLAP is measured as the mass of analyte actually introduced into the chromatographic column. Ten analytical standards were prepared in equal descending increments with the highest standard containing 2.573 µg/mL of desflurane. This is the concentration that would produce a peak approximately 10 times the background noise of a reagent blank near the elution time of the analyte. These standards, and the reagent blank, were analyzed with the recommended analytical parameters (1-µL injection with a 15.5 : 1 split), and the data obtained were used to determine the required parameters (A and SEE) for the calculation of the DLAP. Values of 3.071 and 13.965 were obtained for A and SEE respectively. DLAP was calculated to be 13.6 pg.
| Table 4.2 Detection Limit of the Analytical Procedure | ||
| concentration | mass on column | area counts |
| (µg/mL) | (pg) | (µV-s) |
|
|
||
| 0 0.257 0.515 0.772 1.029 1.286 1.544 1.801 2.058 2.315 2.573 |
0 16.58 33.23 49.81 66.39 82.97 99.61 116.2 132.8 149.4 166.0 |
0 48.2 120.7 154.9 196.2 285.9 291.3 365.8 424.7 460.4 505.8 |
|
| ||
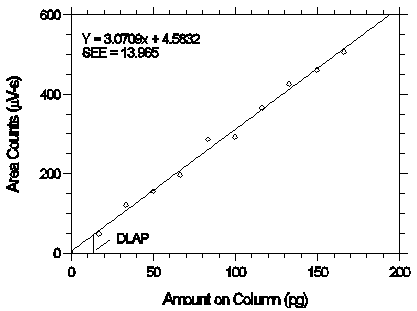
Figure 4.2. Plot of the data from Table 4.2 to determine the DLAP of desflurane.
4.3 Detection limit of the overall procedure (DLOP)
The DLOP is measured as mass per sample and expressed as equivalent air concentration, based on the recommended sampling parameters. Ten samplers were spiked with equal descending increments of analyte, such that the highest sampler loading was 2.573 µg/sample. This is the amount spiked on a sampler that would produce a peak approximately 10 times the background response for a sample blank. These spiked samplers, and a sample blank, were analyzed with the recommended analytical parameters, and the data obtained used to calculate the required parameters (A and SEE) for the calculation of the DLOP. Values of 200.32 and 13.69 were obtained for A and SEE, respectively. The DLOP was calculated to be 0.205 µg/sample (9.93 ppb or 68.4 µg/m3).
| Table 4.3 Detection Limit of the Overall procedure | |
| mass per sample (µg) |
area counts (µV-s) |
|
| |
| 0 0.257 0.515 0.772 1.029 1.286 1.544 1.801 2.058 2.315 2.573 |
0 61.8 113.7 144.1 192.2 265.5 305.5 342.7 396.8 485.3 523.0 |
|
| |
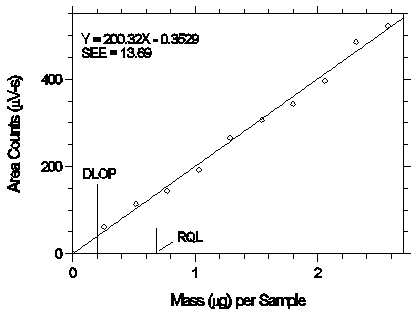
Figure 4.3. Plot of the data to determine the DLOP.
4.4 Reliable quantitation limit (RQL)
The RQL is considered the lower limit for precise quantitative measurements. It is determined from the regression line parameters obtained for the calculations of the DLOP (Section 4.3), providing at least 75% of the analyte is recovered. The RQL is defined as the amount of analyte that gives a response (YRQL) such that
therefore
| RQL = | A |
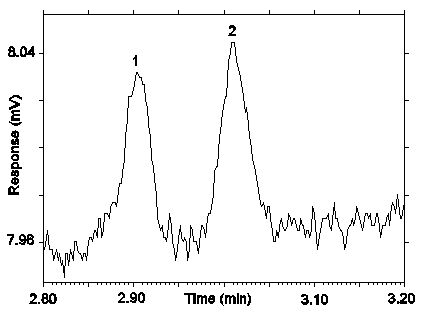
Figure 4.4. Chromatogram of the RQL for desflurane on Anasorb 747.
Peak identification: (1) contaminant form Anasob 747, (2) desflurane.
The RQL for desflurane was calculated to be 0.683 µg/sample (33.1 ppb or 228 µg/m3). The recovery at this concentration is 91.2%.
4.5 Precision (analytical method)
The precision of the analytical procedure is measured as the pooled relative standard deviation (RSDP). Relative standard deviations are determined from six replicate injections of desflurane standards at 0.5, 0.75, 1, 1.5 and 2 times the target concentrations. After assuring that the RSDs satisfy the Cochran test for homogeneity at the 95% confidence level, RSDP was calculated to be 0.58% and 0.46% for the lower and higher target concentration, respectively.
| Table 4.5.1 Instrument Response to Desflurane at Low TC | |||||
| × target concn (µg/mL) |
0.5× 10.29 |
0.75× 15.44 |
1× 20.58 |
1.5× 30.87 |
2× 41.16 |
|
| |||||
| area counts (mV-s) |
2.391 2.394 2.381 2.416 2.417 2.388 |
3.554 3.559 3.549 3.548 3.550 3.530 |
4.743 4.746 4.703 4.729 4.716 4.763 |
6.747 6.777 6.827 6.763 6.863 6.880 |
8.930 8.847 8.793 8.917 8.906 8.886 |
SD RSD (%) |
2.398 0.015 0.63 |
3.548 0.010 0.28 |
4.733 0.022 0.46 |
6.810 0.055 0.81 |
8.880 0.051 0.57 |
|
| |||||
| Table 4.5.2 Instrument Response to Desflurane at High TC | |||||
| × target concn (µg/mL) |
0.5× 774 |
0.75× 1161 |
1× 1547 |
1.5× 2321 |
2× 3095 |
|
| |||||
| area counts (mV-s) |
182.784 182.971 181.180 180.916 182.850 180.309 |
271.647 273.385 271.537 270.495 270.950 270.262 |
361.916 360.981 360.914 358.954 359.275 358.821 |
520.504 523.876 527.304 523.414 524.745 524.994 |
697.847 699.750 693.565 696.571 700.723 694.286 |
SD RSD (%) |
181.835 1.168 0.64 |
271.379 1.126 0.41 |
360.144 1.293 0.36 |
524.140 2.233 0.43 |
697.124 2.877 0.41 |
|
| |||||
The Cochran test for homogeneity:
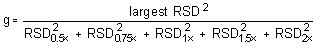
The critical value of the g-statistic, at the 95% confidence level, for five variances, each associated with six observations is 0.5065. The g-statistics are 0.3915 and 0.3773 for the low and high target concentrations respectively. Because the g-statistic does not exceed this value, the RSDs can be considered equal and they can be pooled (RSDP) to give an estimated RSD for the concentration range studied.
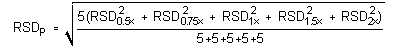
The (RSDP)s are 0.58% and 0.46% for the low and high target concentration respectively.
4.6 Precision (overall procedure)
The precision of the overall procedure is determined from the storage data in Section 4.7. The determination of the standard error of estimate (SEER) for a regression line plotted through the graphed storage data allows the inclusion of storage time as one of the factors affecting overall precision. The SEER is similar to the standard deviation, except it is a measure of the dispersion of data about a regression line instead of about a mean. It is determined with the following equation:
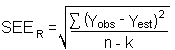
| Yobs | = | observed % recovery at a given time |
| Yest | = | estimated % recovery from the regression line at the same given time |
| n | = | total number of data points |
| k | = | 2 for linear regression |
| k | = | 3 for quadratic regression |
An additional 5% for pump error (SP) is added to the SEER by the addition of variances to obtain the total standard error of the estimate.

The precision at the 95% confidence level is obtained by multiplying the standard error of estimate (with pump error included) by 1.96 (the z-statistic from the standard normal distribution at the 95% confidence level). The 95% confidence intervals are drawn about their respective regression lines in the storage graphs, as shown in Figures 4.7.1.1 through 4.7.2.2. The precisions of the overall procedure are 10.4% and 11.0% for the low and high target concentration, respectively.
4.7 Storage test
- 4.7.1 Storage test at the low target concentration
Storage samples were generated by spiking Anasorb 747 tubes with a toluene solution containing desflurane while pulling air through the tubes at 0.05 L/min. The relative humidity was approximately 80% at 22°C. Humid air was then pulled through the tubes for 30 min. Thirty-six storage samples were prepared. Six samples were analyzed immediately after generation, fifteen tubes were stored at reduced temperature (4°C) and the other fifteen were stored in the dark at ambient temperature (about 22°C). At 2-5 day intervals, three samples were selected from each of the two sets and analyzed.
| Table 4.7.1 Storage Test at the Low TC | ||||||
| time (days) |
ambient storage recovery (%) |
refrigerated storage recovery (%) | ||||
|
| ||||||
| 0 5 8 11 13 18 |
90.2 103.9 97.0 98.3 95.8 101.9 96.7 |
102.0 102.3 101.9 102.6 102.1 98.5 99.0 |
101.2 102.1 99.6 104.2 102.5 100.2 100.8 |
90.2 103.9 101.9 105.3 100.1 103.0 102.3 |
102.0 102.3 102.3 106.0 101.9 102.1 100.2 |
101.2 102.1 101.8 107.0 102.5 103.2 100.6 |
|
| ||||||
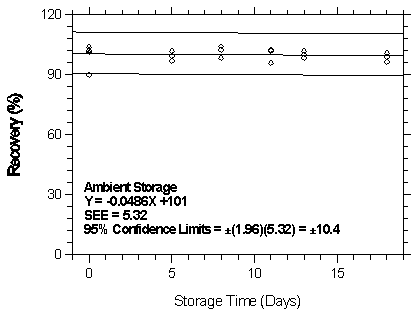
Figure 4.7.1.1. Ambient storage test at 1 ppm.
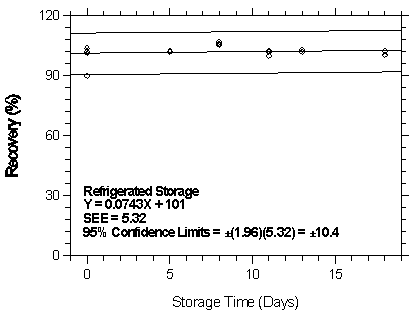
Figure 4.7.1.2. Refrigerated storage test at 1 ppm.
4.7.2 Storage test at the high target concentration
Storage samples were generated by sampling from a controlled test atmosphere containing 1151.2 mg/m3 of desflurane, about 2 times the target concentration. Anasorb 747 tubes were used to sample for 30 min at 0.05 L/min. The relative humidity was approximately 80% at 22°C. Thirty-six storage samples were prepared. Six samples were analyzed immediately after generation, fifteen tubes were stored at reduced temperature (4°C) and the other fifteen were stored in the dark at ambient temperature (about 22°C). At 2-5 day intervals, three samples were selected from each of the two sets and analyzed.
| Table 4.7.2 Storage Test at the High TC | ||||||
| time (days) |
ambient storage recovery (%) |
refrigerated storage recovery (%) | ||||
|
| ||||||
| 0 3 8 11 14 16 |
87.7 102.3 94.8 106.1 94.0 111.4 94.5 |
102.6 105.3 103.4 108.2 107.5 105.0 104.0 |
96.7 106.0 97.2 101.5 94.9 104.6 95.2 |
87.1 102.3 105.4 90.2 110.9 94.4 104.7 |
102.6 105.3 110.0 107.0 104.8 101.4 107.5 |
96.7 106.0 100.8 94.0 105.5 96.9 113.0 |
|
| ||||||
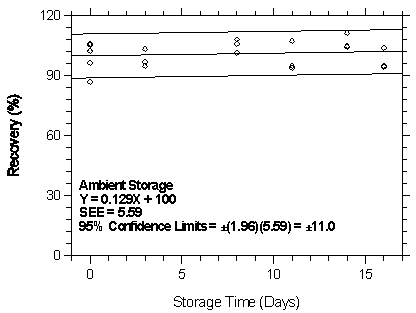
Figure 4.7.2.1. Ambient storage test at 75 ppm.
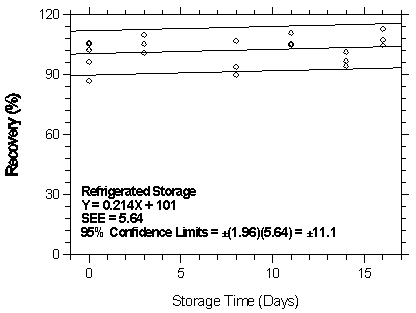
Figure 4.7.2.2. Refrigerated storage test at 75 ppm.
4.8 Reproducibility
- 4.8.1 Reproducibility at low target concentration
Six samples were prepared by injecting microliter quantities of a toluene solution containing desflurane into Anasorb 747 tubes while pulling air through the tubes at 0.05 L/min. The relative humidity was approximately 80% at 22°C. Humid air was then pulled through the tubes for 60 min. The samples were submitted to an OSHA Salt Lake Technical Center service branch. The samples were analyzed after being stored for 10 days at 4°C. Sample results were corrected for desorption efficiency. No sample result for desflurane had a deviation greater than the precision of the overall procedure determined in Section 4.6, which is ±10.4%.
| Table 4.8.1 Reproducibility Data at Low TC | ||||
| sample |
expected (mg/m3) |
reported (mg/m3) |
recovery (%) |
deviation (%) |
|
| ||||
| 1 2 3 4 5 6 |
6.18 6.18 6.18 6.18 6.18 6.18 |
5.77 5.94 5.87 5.87 6.00 6.03 |
93.4 96.1 95.0 95.0 97.1 97.6 |
-6.6 -3.9 -5.0 -5.0 -2.9 -2.4 |
|
| ||||
4.8.2 Reproducibility at high target concentration
Six samples were prepared by injecting microliter quantities of a toluene solution containing desflurane into Anasorb 747 tubes while pulling air through the tubes at 0.05 L/min. The relative humidity was approximately 80% at 22°C. Humid air was then pulled through the tubes for 60 min. The samples were submitted to an OSHA Salt Lake Technical Center service branch. The samples were analyzed after being stored for 10 days at 4°C. Sample results were corrected for desorption efficiency. No sample result for desflurane had a deviation greater than the precision of the overall procedure determined in Section 4.6, which is ±11.0%.
| Table 4.8.2 Reproducibility Data at High TC | ||||
| sample |
expected (mg/m3) |
reported (mg/m3) |
recovery (%) |
deviation (%) |
|
| ||||
| 1 2 3 4 5 6 |
489.4 489.4 489.4 489.4 489.4 489.4 |
451.0 457.6 453.4 448.4 451.0 453.3 |
92.1 93.5 92.6 91.6 92.1 92.6 |
-7.9 -6.5 -7.4 -8.4 -7.9 -7.4 |
|
| ||||
4.9 Sampler capacity
The sampling capacity of the front section of an Anasorb 747 sampling tube was tested by sampling from a dynamically generated test atmosphere of desflurane (1030 mg/m3 or 150 ppm). The samples were collected at 0.05 L/min and the relative humidity was approximately 80% at 22°C. A GC with a gas sampling valve was placed in-line behind the 140-mg front test section. The valve was rotated to measure the amount of desflurane passing through the sampler at the time of rotation. The 5% breakthrough air volume was determined to be 3.83 L.
| Table 4.9 Capacity of Desflurane on Anasorb 747 | |||
| first test | second test | ||
| air volume (L) |
breakthrough (%) |
air volume (L) |
breakthrough (%) |
|
| |||
| 1.60 2.58 3.35 3.87 4.39 |
0 0 0 1.26 37.3 |
1.78 2.72 3.46 3.95 4.56 |
0 0 0 1.56 34.2 |
|
| |||
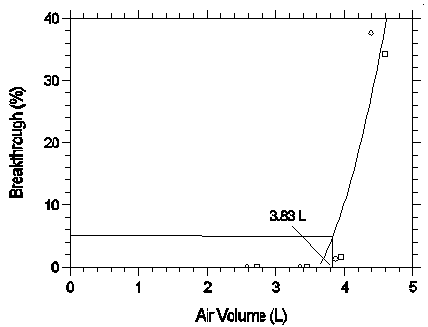
Figure 4.9. Five percent breakthrough air volume for
desflurane on Anasorb 747.
4.10 Desorption efficiency and stability of desorbed samples
- 4.10.1 Anasorb 747 at low target concentration (TC)
The desorption efficiencies (DE) of desflurane were determined by liquid-spiking 140-mg portions of Anasorb 747 with amounts equivalent to 0.05 to 2 times the target concentration. These samples were stored overnight at ambient temperature and then desorbed and analyzed. The average desorption efficiency over the working range of 0.5 to 2 times the target concentration is 101.1%.
| Table 4.10.1.1 Desorption Efficiency of desflurane from Anasorb 747 at Low TC | ||||||
| × target concn (µg/sample) |
0.05× 1.029 |
0.1× 2.058 |
0.2× 4.116 |
0.5× 10.29 |
1.0× 20.58 |
2.0× 41.16 |
|
| ||||||
| DE (%) | 96.6 96.2 98.2 100.1 95.9 101.0 |
100.7 101.9 104.3 103.3 104.8 104.0 |
100.9 100.0 99.1 100.4 100.7 99.9 |
101.4 102.0 101.4 100.7 99.8 100.7 |
102.3 103.2 102.3 101.1 102.8 103.4 |
99.8 100.8 99.0 99.5 99.2 99.8 |
| 98.0 | 103.2 | 100.2 | 101.0 | 102.5 | 99.7 | |
|
| ||||||
The stability of desorbed samples was investigated by reanalyzing the target concentration samples 20 h after initial analysis. After the original analysis was performed, three vials were recapped with new septa while the remaining three retained their punctured septa. The samples were reanalyzed with fresh standards. The average percent change was 2.4% for samples that were resealed with new septa and 0.9% for those that retained their punctured septa.
| Table 4.10.1.2 Stability of Desorbed Samples from Anasorb 747 | |||||
| punctured septa replaced | punctured septa retained | ||||
| initial DE (%) |
DE after one day (%) |
difference | initial DE (%) |
DE after one day (%) |
difference |
|
| |||||
| 102.3 103.2 102.3 102.6 |
100.5 99.4 100.6 averages 100.2 |
-1.8 -3.8 -1.7 -2.4 |
101.1 102.8 103.4 102.4 |
101.7 101.3 101.6 averages 101.5 |
+0.6 -1.5 -1.8 -0.9 |
|
| |||||
4.10.2 Anasorb 747 at high target concentration (TC)
The desorption efficiencies (DE) of desflurane were determined by liquid-spiking 140-mg portions of Anasorb 747 with amounts equivalent to 0.05 to 2 times the target concentration. These samples were stored overnight at ambient temperature and then desorbed and analyzed. The average desorption efficiency over the working range of 0.5 to 2 times the target concentration is 102.9%.
| Table 4.10.2.1 Desorption Efficiency of Desflurane from Anasorb 747 at High TC | ||||||
| × target concn (µg/sample) |
0.05× 77.37 |
0.1× 154.7 |
0.2× 309.5 |
0.5× 773.7 |
1.0× 1547 |
2.0× 3095 |
|
| ||||||
| DE (%) | 99.3 100.4 100.2 99.7 100.5 100.1 |
99.4 100.0 100.0 100.6 101.0 99.9 |
101.6 101.2 100.4 97.4 103.1 102.0 |
105.4 102.9 104.5 103.8 105.2 103.7 |
104.7 102.6 103.0 102.6 103.6 103.4 |
102.0 101.5 101.2 101.4 100.4 100.3 |
| 100.0 | 100.2 | 101.0 | 104.3 | 103.3 | 101.1 | |
|
| ||||||
The stability of desorbed samples was investigated by reanalyzing the target concentration samples 33 h after initial analysis. After the original analysis was performed, three vials were recapped with new septa while the remaining three retained their punctured septa. The samples were reanalyzed with fresh standards. The average percent change was 0% for samples that were resealed with new septa and +0.6% for those that retained their punctured septa.
| Table 4.10.2.2 Stability of Desorbed Samples for Desflurane from Anasorb 747 | |||||
| punctured septa replaced | punctured septa retained | ||||
| initial DE (%) |
DE after one day (%) |
difference | initial DE (%) |
DE after one day (%) |
difference |
|
| |||||
| 104.7 102.6 103.0 103.4 |
103.5 103.4 103.2 (averages) 103.4 |
-1.2 +0.8 +0.2 0 |
102.6 103.6 103.4 103.2 |
104.3 104.4 102.6 (averages) 103.8 |
+1.7 +0.8 -0.8 +0.6 |
|
| |||||
4.11 Qualitative analysis
The mass spectrum for desflurane was obtained from an HP5988A Mass Spec interfaced to a Hewlett-Packard 5890 Series II GC.
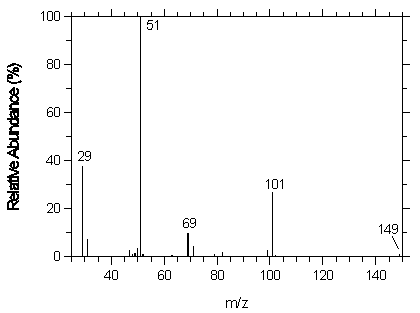
Figure 4.11. Mass spectrum of desflurane.
- 5.1 Burright, D.D. OSHA Method No. 103; Enflurane, Halothane
and Isoflurane, OSHA Salt Lake Technical Center, unpublished, Salt
Lake City, UT 84165, May 1994.
5.2 NIOSH Criteria for a Recommended Standard: Occupational
Exposure to Waste Anesthetic Gases and Vapors, U.S. Department of
Health and Human Services, Public Health Service, Center for Disease
Control, National Institute for Occupational Safety and Health,
Cincinnati, OH, 1977, DHHS (NIOSH) Publ.
5.3 NIOSH Recommendations for Occupational Safety and Health:
Compendium of Policy Documents and Statements, U.S. Department of
Health and Human Services, Public Health Service, Center for Disease
Control, National Institute for Occupational Safety and Health,
Cincinnati, OH, 1992, DHHS (NIOSH) Publ.
5.4 Material Safety Data Sheet: Suprane™, Anaquest, Liberty Corner, NJ, March 1992.
5.5 OSHA Instruction CPL 2-2.60, Exposure Control Plan for Federal OSHA Personnel with Occupational Exposure to Bloodbourne Pathogens, March 7, 1994; Occupational Safety and Health Administration, U.S. Department of Labor, Washington, D.C.