
ETHYLENE GLYCOL
| Method number: | PV2024 |
| Matrix: | Air |
| Target concentration: ACGIH TLV: (Aerosol) |
25 mg/m3 (9.85 ppm) 100 mg/m3 Ceiling |
| Procedure: | Samples are collected by drawing a known volume of air through OSHA Versatile Sampler tubes, containing a glass fiber filter (GFF) and two sections of XAD-7 adsorbent (OVS-7). Samples are desorbed with methanol and analyzed by gas chromatography (GC) using a flame ionization detector (FID). |
| Recommended air volumes and sampling rate: |
60 L at 1.0 L/min (TWA) 15 L at 1.0 L/min (ceiling samples) |
| Reliable quantitation limit: | 0.054 ppm (0.14 mg/m3) |
| Status of method: | Partially Evaluated Method. This method has been subjected to established evaluation procedures, and is presented for information and trial use. |
| Date: February 1999 | Chemist: Wayne Potter |
Chromatography Team
OSHA Salt Lake Technical
Center
Salt Lake City, UT 84115-1802
1. General Discussion
- 1.1 Background
- 1.1.1 History
Airborne ethylene glycol has been determined by using a sampler consisting of a 13-mm glass fiber filter in a Swinnex cassette and a silica gel tube connected in series. Analysis was preformed by gas chromatograph with a flame ionization detector as discribed in NIOSH Method 5500 (Ref. 5.1). This method required the glass fiber filter to be extracted in the field with 2:98 isopropanol:water. Due to excessive sample loss the NIOSH method for ethylene glycol was replaced in 1996 with NIOSH Method 5523 (Ref. 5.2). This method uses the XAD-7 OVS tube which combines the glass fiber filter and XAD-7 into one sampler and is also analyzed by gas chromatography with a flame ionization detector. Method 5523 requires samples to be packed in dry ice for shipment. One of the advantages of Method 5523 is not having to connect the glass fiber filter and the silica gel tube in series. Another advantage is not having to extract the glass fiber filter in the field with the isopropanol:water solution.
The purpose of this study was to evaluate the collection and analysis of ethylene glycol at the ACGIH Ceiling level using a 15 L air sample and the TWA level of 25 mg/m3 using a 60 L air sample.
1.1.2 Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
Ethylene glycol is a human poison by ingestion (Lethal dose for humans reported to be 100 mL). Lower doses are moderately toxic to humans by ingestion, subcutaneous, intravenous, and intramuscular routes. Human systemic effects by ingestion and inhalation include: eye lacrimation, general anesthesia, headache, cough, respiratory stimulation, nausea or vomiting, pulmonary, kidney and liver changes. If ingested it causes initial central nervous system stimulation followed by depression. Later, it causes potentially lethal kidney damage. It is very toxic in the particulate form upon inhalation. Ethylene glycol is an experimental teratogen and has shown other experimental reproductive effects. Ethylene glycol is a skin, eye, and mucous membrane irritant (Ref. 5.3). Concentrations of about 80 ppm or more were intolerable to humans, with a burning sensation along the trachea and a burning cough (Ref. 5.4).
1.1.3 Workplace exposure (Ref. 5.4)
Ethylene glycol is used as an antifreeze in heating and cooling systems, as an industrial humectant, and as a solvent in the paint and plastics industries.
The low vapor pressure of ethylene glycol virtually precludes excessive exposure to the vapors at room temperature. Exposure to vapor and mists of ethylene glycol is possible, however, at elevated temperatures, and adverse effects have been reported from exposure to mists.
1.1.4 Physical properties and other descriptive information (Ref. 5.3 unless otherwise indicated).
| Synonyms: | |
| CAS number: | 107-21-1 |
| IMIS: | 1911 |
| RTECS: | KW 2975000 (Ref. 5.5) |
| Molecular weight: | 62.08 |
| Boiling point: | 197.5°C |
| Melting point: | -13°C |
| Flash point: | 232°F (CC) |
| Vapor pressure: | 0.05 mm @ 20°C |
| Density: | 1.113 @ 25°C / 25°C |
| Properties: | Colorless, sweet-tasting, hygroscopic liquid. |
| Solubility: | Miscible with water and alcohol; slightly soluble in ether; insoluble in benzene and its homologs, chlorinated hydrocarbons, and petroleum ethers. |
| Molecular formula: | C2H6O2 |
| Structural formula: |  |
The analyte air concentrations throughout this method are based on the recommended sampling and analytical parameters. Air concentrations listed in ppm are referenced to 25°C and 101.3 kPa (760 mmHg).
- 1.2 Limit defining parameters
- 1.2.1 Detection limit of the overall procedure (DLOP)
The detection limit of the overall procedure is 2.48 µg per sample (0.016 ppm or 0.041 mg/m3). This is the amount of analyte spiked on the sampler that will give a response that is significantly different from the background response of a sampler blank.
The DLOP is defined as the concentration of analyte that gives a response (YDLOP) that is significantly different (three standard deviations (SDBR)) from the background response (YBR).
The direct measurement of YBR and SDBR in chromatographic methods is typically inconvenient, and difficult because YBR is usually extremely low. Estimates of these parameters can be made with data obtained from the analysis of a series of samples whose responses are in the vicinity of the background response. The regression curve obtained for a plot of instrument response versus concentration of analyte will usually be linear. Assuming SDBR and the precision of data about the curve are similar, the standard error of estimate (SEE) for the regression curve can be substituted for SDBR in the above equation. The following calculations derive a formula for the DLOP:
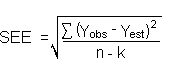
| Yobs | = | observed response |
| Yest | = | estimated response from regression curve |
| n | = | total no. of data points |
| k | = | 2 for a linear regression curve |
At point YDLOP on the regression curve
| YDLOP = A(DLOP) + YBR | A = analytical sensitivity (slope) |
therefore
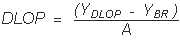
Substituting 3(SEE) + YBR for YDLOP gives
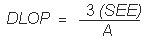
The DLOP is measured as mass per sample and expressed as equivalent air concentrations, based on the recommended sampling parameters. Ten samplers were spiked with equal descending increments of analyte, such that the lowest sampler loading was 2.92 µg/sample. This is the amount, when spiked on a sampler, that would produce a peak approximately 10 times the background response for the sample blank. These spiked samplers, and the sample blank were analyzed with the recommended analytical parameters, and the data obtained used to calculate the required parameters (A and SEE) for the calculation of the DLOP. Values of 359.43 and 296.97 were obtained for A and SEE respectively. DLOP was calculated to be 2.48 µg/sample (0.016 ppm or 0.041 mg/m3).
Detection Limit of the Overall Procedure
|
|
|
| mass per
sample (µg) |
area
counts (µV-s) |
|
| |
| 0 2.92 5.83 8.75 11.67 14.58 17.50 20.41 23.33 26.25 29.16 |
0 623 1428 2309 3271 4469 5597 6668 8163 9079 10044 |
|
| |
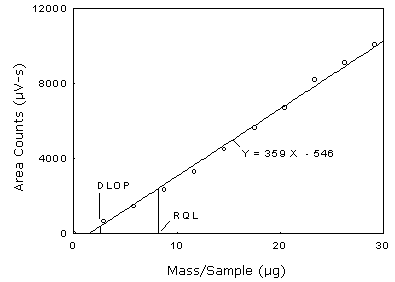
Figure 1.2.1 Plot of data to determine the DLOP/RQL.
1.2.2 Reliable quantitation limit (RQL)
The reliable quantitation limit is 8.26 µg per sample (0.054 ppm or 0.14 mg/m3). This is the amount of analyte spiked on a sampler that will give a signal that is considered the lower limit for precise quantitative measurements.
The RQL is determined from the regression line data obtained for the calculation of the DLOP (Section 1.2.1), providing at least 75% of the analyte is recovered. The RQL is defined as the concentration of analyte that gives a response (YRQL) such that
therefore
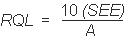
RQL = 8.26 µg per sample (0.14 mg/m3)
The recovery at the RQL is 100%.
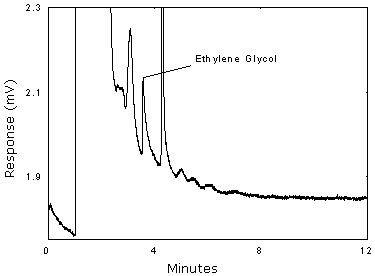
Figure 1.2.3 Chromatogram of the RQL.
2. Sampling Procedure
- 2.1 Apparatus
- 2.1.1 Samples are collected using a personal sampling pump
calibrated, with the sampling device attached, to within ±5% of the
recommended flow rate.
2.1.2 Samples are collected using OVS-7 tubes, which are specially made 11-mm i.d. × 13-mm o.d. × 5.0 cm long glass tubes that taper to 6-mm o.d. × 2.5 cm. Each tube is packed with a 140-mg back section and a 270-mg front section of XAD-7 and a 13-mm diameter glass fiber filter. The back section is retained by two foam plugs and the sampling section is between one foam plug and the glass fiber filter. The glass fiber filter is held next to the sampling section by a polytetrafluoroethylene (PTFE) retainer. These tubes are commercially available from SKC Inc. (catalog no. 226-57) and from both Supelco and Forest Biomedical as a custom product.
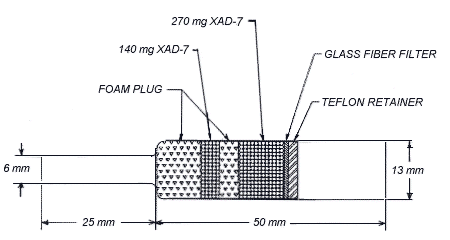
2.2 Technique
- 2.2.1 Immediately before sampling, remove the caps from the
sampling tubes. All tubes should be from the same lot.
2.2.2 Attach the small end of the sampling tube to the pump with flexible tubing. Position the tube so that sampled air passes through the front section (GFF) of the tube first.
2.2.3 Air being sampled should not pass through any hose or tubing before entering the sampling tube.
2.2.4 Attach the sampler vertically with the open end pointing downward, in the worker's breathing zone, and positioned so it does not impede work performance or safety.
2.2.5 After sampling for the appropriate time, remove the sample and seal the tube with plastic end caps. Wrap each sample end-to-end with a Form OSHA-21 seal.
2.2.6 Submit at least one blank sample with each set of samples. Handle the blank sampler in the same manner as the other samples except draw no air through it.
2.2.7 Record the sample volume (in liters of air) for each sample, along with any potential interferences.
2.2.8 Ship any bulk samples in separate containers from the air samples.
2.2.9 Submit the samples to the laboratory for analysis as soon as possible after sampling. If delay is unavoidable, store the samples in a refrigerator.
2.3 Extraction and desorption efficiency
The extraction and desorption efficiencies of ethylene glycol were determined by liquid-spiking the 13-mm glass fiber filters and also an amount of XAD-7 adsorbent equal to the adsorbing section (270 mg) of an OVS-7 tube with the analyte at 0.1 to 2 times the target concentration. The loadings on the glass fiber filters and the tubes were 145.8, 729.1, 1458.2, and 2916.4 µg of ethylene glycol. These samples were stored overnight at ambient temperature and then desorbed and analyzed by GC-FID. The average extraction efficiency over the studied range was 99.8% for GFF and 99.5% for XAD-7.
Extraction Efficiency of Ethylene Glycol From GFF
|
| ||||
| Tube # | % Recovered | |||
| 0.1 × 145.8 µg |
0.5 × 729.1 µg |
1.0 × 1458.2 µg |
2.0 × 2916.4 µg | |
|
| ||||
| 1 2 3 4 5 6  overall  SD |
102.6 103.2 102.1 101.9 101.5 101.2 102.1 99.8 ± 1.67 |
98.7 96.0 97.3 97.3 98.6 97.5 97.6 |
98.3 99.3 100.2 98.8 99.4 98.4 99.1 |
100.9 101.4 100.8 100.5 100.1 99.3 100.5 |
|
| ||||
Desorption Efficiency of Ethylene Glycol From XAD-7
|
| ||||
| Tube # | % Recovered | |||
| 0.1 × 145.8 µg |
0.5 × 729.1 µg |
1.0 × 1458.2 µg |
2.0 × 2916.4 µg | |
|
| ||||
| 1 2 3 4 5 6  overall  SD |
100.9 99.2 101.3 102.6 101.8 102.6 101.4 99.5 ± 2.29 |
95.5 96.4 96.1 96.2 95.6 95.2 95.8 |
99.4 98.7 99.0 99.6 99.7 99.3 99.3 |
100.1 101.9 101.9 102.1 100.9 101.7 101.4 |
|
| ||||
2.4 Retention efficiency
The glass fiber filter of six sampling tubes was physically separated from the XAD-7 resin beads and placed on top of the PTFE retainer in each tube to prevent wicking. The glass fiber filters were spiked with 2916.4 µg (194.4 mg/m3 based on a 15 L air sample) ethylene glycol, allowed to equilibrate overnight at room temperature, and then 60 L humid air (80% RH at 25°C) was drawn through them at 1.0 L/min. The sampling tubes were opened and the GFF, the front section and the back section were each put in separate vials. The samples were desorbed and analyzed by GC-FID. The retention efficiency averaged 99.8%.
Retention Efficiency of Ethylene Glycol
|
| ||||
| Tube # | % Recovered | |||
| GFF | Front Section | Back Section | Total | |
|
| ||||
| 1 2 3 4 5 6 |
58.3 36.5 37.8 46.0 32.0 49.3 |
40.4 60.9 60.1 54.1 66.1 48.3 |
1.1 1.8 1.7 0.9 1.9 1.6  |
99.8 99.2 99.6 101.1 100.0 99.2 99.8 |
|
| ||||
2.5 Sample storage
The glass fiber filters of twenty-four sampling tubes were each spiked with 1458.2 µg (97.2 mg/m3 based on a 15 L air sample) of ethylene glycol. They were sealed and stored at room temperature. The next day 60 L of humid air (80% RH at 25°C) was drawn through each tube at 1.0 L/min. Half of the tubes were stored in a drawer at ambient temperature and the other half were stored in a refrigerator at 0°C. After 10 days of storage six samples from the tubes stored under refrigeration and six samples from ambient storage were analyzed. The remaining samples were analyzed after 17 days of storage. The average recovery of the ambient and refrigerated storage samples was 97.5%.
Storage Test for Ethylene Glycol
|
| ||||
| Ambient Storage | | | Refrigerated Storage | ||
| Time (days) | % Recovered | | | Time (days) | % Recovered |
|
| ||||
| 10 10 10 10 10 10 17 17 17 17 17 17  |
98.7 99.2 99.3 97.1 99.6 99.4 96.1 95.4 96.5 96.8 95.8 97.2 97.6 |
| | | | | | | | | | | | | |
10 10 10 10 10 10 17 17 17 17 17 17  |
99.3 97.9 98.7 97.8 96.8 96.9 96.6 95.6 97.9 96.9 96.1 98.0 97.4 |
|
| ||||
2.6 Recommended air volume and sampling rate.
Based on the data collected in this evaluation, 15 L air samples should be collected at a sampling rate of 1.0 L/min for ceiling samples and 60 L at 1.0 L/min for TWA samples.
2.7 Interferences (sampling)
- 2.7.1 It is not known if any compounds will severely interfere
with the collection of ethylene glycol on OVS-7 sampling tubes. In
general, the presence of other contaminants in the air will reduce
the capacity of the sampling tube to collect ethylene glycol.
2.7.2 Suspected interferences should be reported to the laboratory with submitted samples.
2.8 Safety precautions (sampling)
- 2.8.1 Attach the sampling equipment to the worker in such a
manner that it will not interfere with work performance or safety.
2.8.2 Follow all safety practices of the Chemical Hygiene Plan that apply to the work area being sampled.
2.8.3 Wear eye protection at all times while in the work areas.
3. Analytical Procedure
- 3.1 Apparatus
- 3.1.1 A gas chromatograph equipped with a flame ionization
detector. A Hewlett Packard model 5890 was used in this study.
3.1.2 A GC column capable of separating the analyte from any interferences. The column used in this study was a 30 meter Rtx-35 fused silica capillary column with a 3.0 µm film thickness and 0.53 mm i.d.
3.1.3 An electronic integrator or some suitable method of measuring peak areas. A Hewlett Packard model 3396A and the Waters Millennium Data System was used in this study.
3.1.4 Four milliliter vials with TeflonTM-lined caps.
3.1.5 A 10-µL syringe or other convenient size for sample injection.
3.1.6 Pipets for dispensing the desorbing solution. A 2-mL Labindustries dispenser was used in this study.
3.1.7 Volumetric flasks - 10-mL and other convenient sizes for preparing standards.
3.1.8 A balance for weighing ethylene glycol in standard preparation. A Ohaus Galaxy 160D was used in this evaluation.
3.2 Reagents
- 3.2.1 GC grade nitrogen, hydrogen and air.
3.2.2 Ethylene glycol (CAS 107-21-1), Reagent grade. Eastman, Lot O3A, was used in this evaluation.
3.2.3 Methanol (CAS 67-56-1), Certified Spectanalyzed grade. Fisher Chemical, Lot 970338, was used in this evaluation.
3.3 Standard preparation
- 3.3.1 At least two separate stock standards are prepared by
weighing a quantity of ethylene glycol and diluting with methanol.
The concentration of these stock standards was approximately 146.0
mg/mL.
3.3.2 Dilutions of these stock standards were prepared to bracket the samples. The range of the standards used in this study was from 72.9 to 1458.2 µg/mL.
3.4 Sample preparation
- 3.4.1 Sample tubes are opened and the front section (GFF and 270
mg adsorbent), and back section of each tube are placed in separate
4-mL vials. Discard foam plugs.
3.4.2 Each section is desorbed with 2 mL of methanol.
3.4.3 The vials are sealed immediately and allowed to extract/desorb for one hour on a mechanical rotator or the vials are shaken vigorously by hand several times during the extraction/desorption time.
3.4.4 Transfer some of the solution from each of the 4-mL vials to smaller glass vials suitable for an autosampler if necessary.
3.5 Analysis
- 3.5.1 Gas chromatograph conditions.
| Injection size: | 1 µL |
| Flow rates (mL/min) | |
| Nitrogen (make-up): | 30 |
| Hydrogen (carrier): | 2 |
| Hydrogen (detector): | 40 |
| Air: | 420 |
| Temperatures (°C) | |
| Injector: | 200 |
| Detector: | 275 |
| Column: | 40 isothermal |
| Chromatogram: | |
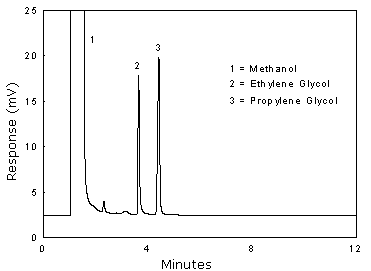 Figure 3.5.1 Chromatogram at the target concentration. | |
3.5.2 Peak areas are measured by an integrator or other suitable means.
3.6 Interferences (analytical)
- 3.6.1 Any compound that produces an FID response and has a
similar retention time as the analyte is a potential interference.
If any potential interferences were reported, they should be
considered before samples are desorbed. Generally, chromatographic
conditions can be altered to separate an interference from the
analyte. Using the analytical conditions in this method, propylene
glycol does not interfere.
3.6.2 When necessary, the identity or purity of an analyte peak may be confirmed by a GC-mass spectrometer or by another analytical procedure.
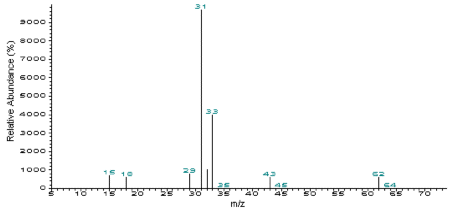
Figure 3.6.2 Mass spectra of ethylene glycol.
3.7 Calculations
- 3.7.1 The calibration curve was made from at least four
standards at different concentrations bracketing the samples.
3.7.2 The values for the samples are obtained from the calibration curve.
3.7.3 To calculate the concentration of analyte in the air sample the following formulas are used:
| (µg/mL) (desorption
volume) (desorption efficiency) |
= mass of analyte in sample |
| (mass of analyte in
sample) molecular weight |
= number of moles of analyte |
| ( | number of moles of analyte | ) | ( | molar volume at
|
) | = | ( | volume the analyte will occupy
at |
) |
| (volume analyte occupies)
(106)* (air volume) |
= ppm |
* All units must cancel.
3.7.4 The above equations can be consolidated to the following formula.
| (µg/mL) (DV)
(24.46) (106) (g)
(mg) (Liters) (DE) (MW) (1000 mg) (1000 µg) |
= ppm |
| µg/mL | = | Concentration of analyte in sample or standard |
| 24.46 | = | Molar volume (liters/mole) at 25°C and 760 mm Hg |
| MW | = | Molecular weight (g/mole) |
| DV | = | Desorption volume |
| Liters | = | Liters of air sample |
| DE | = | Desorption efficiency |
3.7.5 To calculate the mg/m3 of analyte in the sample based on a 60 liter air volume:
| (µg/mL) (DV) (mg) (1000
L) (Liters) (DE) (1000 µg) (m3) |
= mg/m3 |
where:
| µg/mL | = | Concentration of analyte in sample or standard |
| DV | = | Desorption volume |
| Liters | = | Liters of air volume |
| DE | = | Desorption efficiency |
3.7.6 This calculation is done for each section of the sampling tube and the results added together after a blank correction is performed, if necessary.
3.8 Safety precautions
- 3.8.1 Adhere to the rules set down in your Chemical Hygiene Plan
(which is mandated by the OSHA laboratory standard).
3.8.2 Avoid skin contact and inhalation of all chemicals.
3.8.3 Wear safety glasses, gloves and a lab coat at all times while in the laboratory areas.
4. Recommendations for Further Study
Collection studies should be performed with controlled test atmospheres. This method does not differentiate between particulate and vapor so more work could be done to find a sampling device that would do that.
5. References
- 5.1 National Institute for Occupational Safety
and Health: Method No. 5500, Ethylene Glycol, in NIOSH Manual of
Analytical Methods. 3rd ed., Vol.1, Cincinnati, OH:
National Institute for Occupational Safety and Health, 1984.
5.2 National Institute for Occupational Safety and Health: Method No. 5523, Glycols, in NIOSH Manual of Analytical Methods. 4th ed., Vol.1, Cincinnati, OH: National Institute for Occupational Safety and Health, 1996.
5.3 Lewis, Richard J. Sr., "Sax's Dangerous Properties of Industrial Materials", Eighth Edition, Van Nostrand Reinhold Co., New York, 1992, Vol. 2, pp. 1604-1605.
5.4 "Documentation of the Threshold Limit Values and Biological Exposure Indices", 6th ed., American Conference of Governmental Industrial Hygienists Inc., Cincinnati, OH, 1991, p. 612.
5.5 Sweet, D., "Registry of Toxic Effects of Chemical Substances", 1985-86 ed., U.S. Department of Health and Human Services, Public Health Service, Center for Disease Control, NIOSH, 1987, Vol. 3, Index Number KW2975000, p.2359.