BROMINE IN WORPLACE ATMOSPHERES
| Method Number: | ID-108 |
| Matrix: | Air |
| OSHA Permissible Exposure Limits | |
| Bromine (Final Rule Limits): | 0.1 ppm (Time Weighted Average) 0.3 ppm (Short-Term Exposure Limit) |
| Bromine (Transitional Limit): | 0.1 ppm (Time Weighted Average) |
| Collection Procedure: | A calibrated personal sampling pump is used to draw a known volume of air through a midget fritted glass bubbler containing a sodium bicarbonate/sodium carbonate buffer solution. |
| Recommended Sampling Rate: | 0.5 L/min |
| Recommended Air Volumes Short-Term Exposure Limit Samples: |
7.5 L |
| Time Weighted Average Samples: | 30 L |
| Analytical Procedure: | In the buffer collection solution, bromine disproportionates to bromide and bromate ions. Samples are analyzed with minimum sample preparation as these anions by ion chromatography. |
| Detection Limits Qualitative: |
0.001 ppm (30-L air volume) |
| Quantitative: | 0.005 ppm (30-L air volume) |
| Precision and Accuracy Validation Level: |
0.052 to 0.21 ppm |
| CVT: | 0.067 |
| Bias: | -0.056 |
| Overall Error: | ±19% |
| Method Classification: | Validated Method |
| Chemist: | James Ku |
| Date (Date Revised): | 1982 (April, 1990) |
Commercial manufacturers and products mentioned in this method are for descriptive use only and do not constitute endorsements by USDOL-OSHA.
Similar products from other sources can be substituted.
Branch of Inorganic Methods Development
OSHA Technical
Center
Salt Lake City, Utah
1. Introduction
This method describes the collection of airborne bromine (Br2) in the breathing zone of personnel in the workplace and the subsequent analysis by ion chromatography (IC).
1.1. History
Previously, Br2 air samples were collected in 0.01 N NaOH and analyzed as bromide (Br¯) at the OSHA Laboratory by ion specific electrode (ISE) after neutralization with nitric acid. This method had lower recoveries than expected (8.1.) and was possibly due to Br¯ and bromate (BrO3¯) reacting with each other and gradually reverting back to Br2 in an acidic solution. According to the following equation (8.2.):
BrO3¯ + 5Br¯ + 6H+ ----> Br2 + 3H2O
loss of analyte can occur in acidic media. The ISE analysis was further hampered by interferences from other ions such as chloride, iodide, cyanide, or ammonia (8.3.). A method for bromine was developed to allow analysis by ion chromatography and to diminish any problems with interferences.
1.2. Principle
Bromine is collected in a midget-fritted glass bubbler (MFGB) containing a weakly basic buffer [sodium carbonate / sodium bicarbonate (Na2CO3 / NaHCO3)]. Bromine disproportionates in basic solution to produce Br¯ and BrO3¯ according to the following equation (8.4.).
3Br2 + 6OH¯ -----> BrO3¯ + 5Br¯ + 3H2O
The mole ratio of Br2 per Br¯ formed is 1.2. The amount of Br¯ measured by IC is multiplied by this factor to gravimetrically convert Br¯ to Br2. The BrO3¯ formed can also be measured, in principle, with this method. Because the BrO3¯ peak may overlap with peaks of chloride or iodate, measurement of BrO3¯ is normally only used to confirm the presence of Br2.
1.3. Advantages and Disadvantages
1.3.1. This method has adequate sensitivity for measuring workplace atmosphere concentrations of Br2.
1.3.2. This method is less affected by interferences found in other methods (i.e. ion specific electrode, Volhard titration, and colorimetric determinations).
1.3.3. The analytical method can be fully automated. Use of an automatic sampler can improve analytical precision.
1.3.4. The amount of Br2 may be simultaneously confirmed from the bromate anion formed during disproportionation.
1.3.5. High humidity does not affect the sampling procedure.
1.3.6. A disadvantage is the sampling device. Use of bubbler collection techniques may impose certain inconveniences. Spillage can occur during sampling, handling, and during transportation to the lab.
1.4. Uses
Bromine is used in the following operations (8.5.):
the manufacture of ethylene dibromide (anti-knock
gasoline)
organic synthesis reactions
bleaching
agents
fumigants, as an intermediate (methyl
bromide)
fire-retardants for plastics, dyes, and
photography
water purification
shrink-proofing wool
1.5. Physical Properties
Bromine (CAS No. 7726-95-6) is a fuming liquid with a pungent odor. Some physical properties of Br2 are listed below (8.5., 8.6.):
| Atomic Number | 35 |
| Atomic Symbol | Br |
| Molecular Weight (Br2) | 159.89 |
| Melting Point | -7.2 °C |
| Boiling Point | 59.4 °C |
| Vapor Pressure | 22.9 kPa (172 mmHg) at 20 °C |
| Color (liquid) | Reddish-brown |
| Flammability | Nonflammable |
| Classification | Corrosive, strong oxidizer |
| Solubility | Slightly soluble in H2O |
2. Range and Detection Limit (8.1.)
The analytical portion of the evaluation was conducted using a model 10
ion chromatograph (Dionex, Sunnyvale CA) with a 3 ×
2.1. The working range was 0.015 to 0.31 ppm (30-L air sample).
2.2. The sensitivity of the method was 1.96 microsiemens/cm/µg as Br¯. The ion chromatograph was set on a range of 30 microsiemens.
2.3. The qualitative detection limit of the analytical method was
0.003 µg of Br¯ per
2.4. The quantitative limit was 0.015 µg Br¯ per
3. Method Performance (8.1.)
This method was evaluated in 1982 using commercial analytical equipment mentioned in Section 2. Advances in ion chromatographic and sampling instruments should enable users to obtain similar or better results as those mentioned below.
3.1. The CV for the overall sampling and analytical method (CVT[pooled]) in the range of 0.052 to 0.205 ppm was 0.067.
3.2. In validation experiments, this method was capable of measurements within ± 25% of the true value over the validation range (95% confidence level). Bias was -0.056 and overall error was ±19%.
3.3. Collection efficiency was 100% for the buffer collection solution.
3.4. A breakthrough test, conducted at a concentration of 0.21 ppm, indicated breakthrough of 2.4% occurred after sampling for 240 min at a sampling rate of 0.5 L/min. This amount of breakthrough is within acceptable limits (<5% breakthrough).
3.5. In storage stability studies, the mean of samples analyzed after 15 days was within 1% of the mean of samples analyzed immediately after collection. After 30 days the mean was within 10%.
4. Interferences
4.1. Any substance that has the same retention time as BrO3¯ or Br¯ using the operating conditions as described in this method is an interference.
4.2. Nitrate is a potential interference if present in concentrations greater than Br¯. Anions such as chloride (Cl¯), chlorate (ClO3¯) and iodate (IO3¯) may interfere with bromate analysis. These interferences may be minimized by changing the eluent strength, eluent pump flow rate, or separator column. A gradient pump system and an alternate eluent (as specified in Section 6.7.) displayed excellent separation of ClO2¯, Cl¯, and BrO3¯. This alternate eluent is recommended when Cl¯ or other anions prevent positive identification of the presence of bromine with the bromate peak.
4.3. Bromide collected with Br2 in air is an interference; however, differentiation of these two species can be accomplished by determining the amount of BrO3¯ in the sample.
5. Sampling
5.1. Equipment
5.1.1. Collection solution, 0.003 M sodium bicarbonate (NaHCO3)/0.0024 M sodium carbonate (Na2CO3): Dissolve 0.25 g each NaHCO3 and Na2CO3 in approximately 250 mL of DI H2O and then dilute to 1 L with DI H2O. Prepare a new solution every month.
5.1.2. Personal sampling pumps capable of sampling within ± 5% of the recommended flow rate of 0.5 L/min are used.
5.1.3. Midget fritted glass bubblers (MFGBs) (25-mL, part no. 7532, Ace Glass Co., Vineland, NJ).
5.1.4. Shipping vials, glass or plastic: Scintillation vials,
20-mL (part no. 74515 or 58515, Kimble, Div. of
5.1.5. A stopwatch and bubble tube or meter are used to calibrate pumps.
5.1.6. Various lengths of polyvinyl chloride (PVC) tubing are used to connect bubblers to the pumps.
5.2. Sampling Procedure
5.2.1. Calibrate each sampling pump with a calibration sampling device in-line. The calibration device is a MFGB containing about 10 mL of collection solution.
5.2.2. Place 10 to 15 mL of fresh collection solution in each MFGB. Connect a MFGB to a calibrated sampling pump and then place the sampling device in the breathing zone of the employee.
5.2.3. Sample at a flow rate of 0.5 L/min. For STEL determinations, sample for at least 15 min. For measurements of TWA exposures, sample up to 240 min. Take enough samples to cover the shift worked.
5.2.4. Transfer the collection solution into a 20-mL glass
scintillation vial. Rinse the bubbler with 2 to 3 mL of unused
collection solution and transfer the rinsings into the sample vial.
Place the
5.2.5. Prepare a blank solution by taking 10 to 15 mL of the
unused collection solution and transfer to a
5.2.6. Request bromine analysis on the OSHA 91A form.
5.2.7. Ship the samples to the laboratory using appropriate packing materials to prevent breakage. The identities of any substances known or suspected to be present in the sampled air should be transmitted with the sample.
6. Analysis
6.1. Precautions
6.1.1. Refer to instrument and Standard Operating Procedure (8.7.) manuals for proper operation.
6.1.2. Observe laboratory safety regulations and practices.
6.1.3. Sulfuric acid (H2SO4) can cause severe burns. Wear protective eyewear, gloves, and labcoat when using concentrated H2SO4.
6.2. Equipment
6.2.1. Ion chromatograph (model no. 2010i or 4500, Dionex, Sunnyvale, CA) equipped with a conductivity detector.
6.2.2. Automatic sampler (model no. AS-1, Dionex) and 0.5 mL sample vials.
6.2.3. Laboratory automation system: Ion chromatograph interfaced to a data reduction and control system (AutoIon 450, Dionex).
6.2.4. Micromembrane suppressor (model no. AMMS-1, Dionex).
6.2.5. Anion separator column (model no. HPIC-AS4A, Dionex) with
pre-column (model no.
6.2.6. Disposable syringes (1 mL).
6.2.7. Syringe pre-filters, 0.5-µm pore size (part no. SLSR 025 NS Millipore Corp., Bedford, MA).
| (Note: | Some syringe pre-filters are not cation- or anion-free. Tests should be done with blank solutions first to determine suitability for the analyte being determined). |
6.2.8. Miscellaneous volumetric glassware: micropipettes, volumetric flasks, graduated cylinders, and beakers.
6.2.9. Analytical balance (0.01 mg).
6.3. Reagents - All chemicals should be at least reagent grade.
6.3.1. Deionized water (DI H2O) with a specific conductance of less than 10 microsiemens.
6.3.2. Sodium carbonate.
6.3.3. Sodium bicarbonate.
6.3.4. Sodium hydroxide (for alternate eluent).
6.3.5. Eluent [0.0015 M sodium carbonate (Na2CO3) / 0.0015 M sodium bicarbonate (NaHCO3)]: Dissolve 0.636 g Na2CO3 and 0.504 g NaHCO3 in 4.0 L of DI H2O.
6.3.6. Alternate eluent: An alternate eluent is used if interferences are unresolvable. For a gradient pump, use a mixture of: 97% DI H2O / 3% of 0.1 M NaOH. If a gradient pump is not available, this is equivalent to 0.003 M NaOH.
6.3.7. Collection solution (0.003 M NaHCO3 / 0.0024 M Na2CO3): See Section 5.1.1. for instructions.
6.3.8. Sulfuric acid (H2SO4), concentrated (98%).
6.3.9. Regeneration solution (0.02 N
H2SO4): Carefully add 1.14 mL concentrated
H2SO4 into a
6.3.10. Standard stock solution, Br¯ (1,000 µg/mL as bromide): Dissolve and dilute 1.4893 g potassium bromide (KBr) to 1 L with DI H2O.
6.3.11. Standard stock solution, BrO3¯ (1,000 µg/mL as bromate): Dissolve and dilute 1.3057 g of potassium bromate (KBrO3) to 1 L with DI H2O.
6.4. Standard Preparation
Standards (10 and 1 µg/mL as Br¯ or BrO3¯): Make appropriate serial dilutions of Br¯ and BrO3¯ stock solutions with the collection solution. Take aliquots of these standards and prepare the working standards in the range of 0.1 to 5 µg/mL using the collection solution as the diluent.
6.5. Sample Preparation
6.5.1. Measure and record the total volume of each sample with a graduated cylinder.
6.5.2. If the sample solutions contain suspended particulate,
remove the particles using a
6.6. Analytical Preparation
6.6.1. Fill the 0.5-mL automatic sampler vials with sample, working standard, or blank solutions and push a filtercap into each vial. Label the vials.
6.6.2. Load the automatic sampler with labeled samples, standards and blanks.
6.7. Analytical Procedure
Set up the ion chromatograph and analyze the samples and standards in accordance with the Standard Operating Procedure (8.7.). Typical operating conditions for a Dionex 2010i with a data reduction system are listed below.
| Standard Conditions | |
| Ion chromatograph
| |
| Eluent: | 0.0015 M Na2CO3 / 0.0015 M NaHCO3 |
| Separator Column: | AS4A |
| Column temperature: | ambient |
| Regenerant flow: | 1 to 3 mL/min |
| Pump
| |
| Pump pressure: | approximately 1,000 psi |
| Flow rate: | 2 mL/min |
| Chromatogram
| |
| Run time: | 6 min |
| Sample injection loop: | 50 µL |
| Average retention time: | |
| Br¯ | approximately 2.5 min |
| BrO3¯ | approximately 1.5 min |
Alternate conditions are available if interference from other anions pose insufficient resolution. Operating conditions with a model 4500 gradient pump system (Dionex) are:
| Alternate Conditions
|
|
| Ion chromatograph
| |
| Eluent: | 97% DI H2O / 3% 0.1 M NaOH |
| Separator Column: | AS5 |
| Column temperature: | ambient |
| Regenerant flow: | 1 to 3 mL/min |
| Pump
| |
| Pump pressure: | approximately 800-1,000 psi |
| Flow rate: | 1 mL/min |
| Chromatogram
| |
| Run time: | 14 min |
| Sample injection loop: | 50 µL |
| Average retention time: | |
| Br¯ | approximately 10.9 min |
| BrO3¯ | approximately 4.3 min |
The alternate conditions should be used when nitrate, chloride, or other anions interfere and make positive identification of bromide and bromate peaks difficult.
7. Calculations
7.1. Hard copies of chromatograms containing peak area and height data can be obtained from a printer. A typical chromatogram of Br¯ and BrO3¯ is shown in Figure 1 and one with interference from chloride on the bromate peak is shown in Figure 2. A chromatogram of 10 µg/mL standards of BrO3¯, ClO2¯, and Br¯ using suggested alternate conditions (Section 6.7.) is shown in Figure 3.
7.2. Prepare a concentration-response curve by plotting the concentration of the standards in µg/mL (or µg/sample if the same volumes are used for samples and standards) versus peak areas or peak heights. Assuming a constant sample injection volume was used for all samples and standards, calculate sample concentrations (total µg) from the curve. Blank correct all samples as shown:
µgC Analyte = (S)(SV) - (BL)(BLV)
| Where: | |||
| µgC Analyte | = | Corrected amount (µg) in the sample solution. | |
| S | = | µg/mL sample (from curve) | |
| SV | = | Sample solution volume, mL (from Section 6.5.1.) | |
| BL | = | µg/mL blank (from curve) | |
| BLV | = | Blank solution volume, mL (from Section 6.5.1.) | |
7.3. The concentration of Br2 in each air sample is expressed in ppm and is calculated as:
| ppm Br2 = | MV × µgC analyte × conversion
formula weight × air volume |
| Where: | |||
| MV (Molar Volume) | = | 24.45 (25 °C and 760 mmHg) | |
| µgC Analyte | = | blank corrected sample result | |
| Conversion (Br¯ to Br2) | = | 1.2 | |
| Conversion (BrO3¯ to Br2) | = | 3.75 | |
| Formula Weight (Br2) | = | 159.8 | |
| Air Volume | = | Air sample taken (in L) | |
This equation reduces to:
| ppm Br2 = | 0.184 × µgC Br¯
air volume |
(for Br¯ as analyte) |
or:
| ppm Br2 = | 0.574 µgC BrO3¯
air volume |
(confirmation using BrO3¯) |
7.4. Reporting Results
Results are reported to the industrial hygienist as ppm bromine. The numerical result is determined from the bromide analysis.
8. References
8.1. Occupational Safety and Health Administration Technical
Center: Bromine Backup Data Report
8.2. Blaedel, W.J. and V.W. Meloche: Elemental Quantitative Analysis -- Theory and Practice. 2nd ed. New York: Harper and Row, Publishers, 1963. p. 854.
8.3. Orion Research Incorporated: Instruction Manual,
Halide Electrodes, Model
8.4. Cotton, F.A. and G. Wilkinson: Advanced Inorganic
Chemistry -- A Comprehensive Text. 2nd rev. ed. New York:
Interscience Publishers, 1966. pp.
8.5. Hawley, G.G.: The Condensed Chemical Dictionary. 11th ed. New York: Van Nostrand Reinhold Co., 1987.
8.6. National Oceanic and Atmospheric Administration (NOAA): CAMEO [Computer Software]. NOAA, 1988.
8.7. Occupational Safety and Health Administration Technical Center: Ion Chromatography Standard Operating Procedure. Salt Lake City, UT. In progress (unpublished).
Chromatogram of Br¯ and BrO3¯ (AS4A Column)
| PEAK NUM |
RET TIME |
PEAK NAME |
AREA | HEIGHT | |||
|
| |||||||
| 1 2 |
1.42 2.48 |
BROMATE BROMIDE |
3.675e+004 5.881e+004 |
5264 5212 | |||
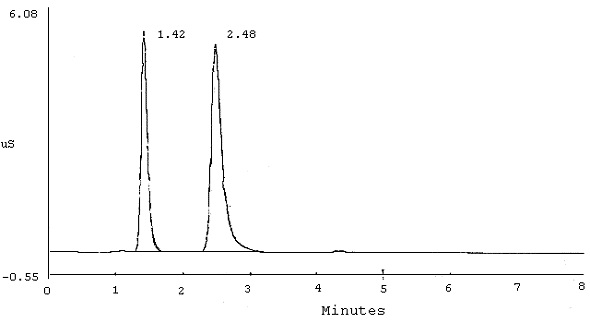
Figure 1
Chromatogram - Mixture if BrO3¯, Cl¯, NO3¯, HPO42¯, and SO42¯ (AS4A Column)
| PEAK NUM |
RET TIME |
PEAK NAME |
AREA | HEIGHT | |||
|
| |||||||
| 1 2 3 4 5 6 |
1.00 1.52 2.48 2.83 3.57 4.33 |
CHLORIDE BROMIDE NITRATE PHOSPHATE SULFATE |
5.088e+003 7.232e+004 1.256e+004 1.801e+004 3.381e+004 1.132e+005 |
572 10323 1485 1680 2864 7023 | |||
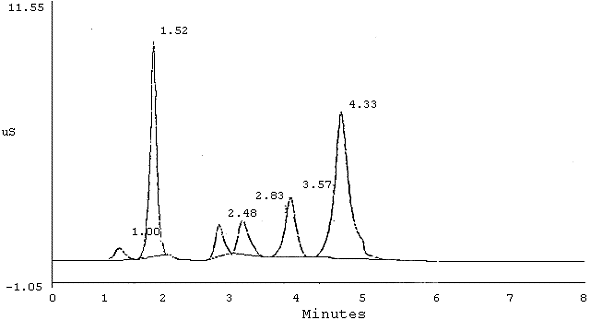
Figure 2
Chromatogram of Mixture of Chlorite, Bromate, Chloride,
and Bromine
(AS5 separator column, gradient pump, and 97% DI
H2O / 3% 0.1 M NaOH eluent)
| PEAK NUM |
RET TIME |
PEAK NAME |
AREA | HEIGHT | |||
|
| |||||||
| 1 2 3 4 5 6 7 8 9 10 11 12 |
0.45 1.68 2.37 2.72 3.15 4.25 4.80 5.80 7.35 9.17 10.88 13.13 |
CHLORITE BROMATE CHLORIDE BROMIDE |
4.794e+005 1.181e+007 1.283e+007 8.283e+005 5.348e+007 6.865e+007 2.031e+008 6.541e+005 2.443e+006 5.396e+005 2.231e+008 3.520e+006 |
21724 559853 1040749 100649 6711421 6814401 18500982 52785 35239 14843 5722599 40073 | |||
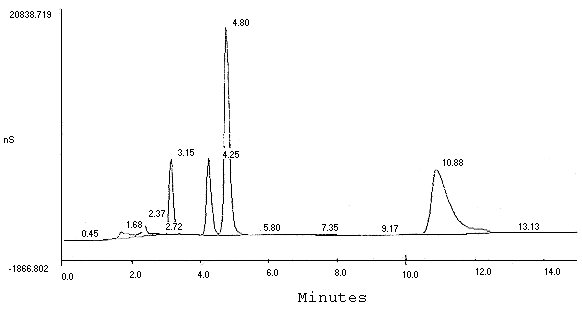
Figure 3