| Method Number: | ID-180 | ||
| Matrix: | Air | ||
| OSHA Permissible Exposure
Limits Final Rule Limit: Transitional Limit: |
0.3 ppm [Time Weighted Average (TWA)] 1 ppm [Short-Term Exposure Limit (STEL)] 0.3 ppm (TWA) | ||
| Collection Device: | Samples are collected using a calibrated sampling pump and a glass tube containing beaded carbon impregnated with potassium hydroxide. A humidification device may also be used in front of the sorbent tube. | ||
| Recommended Sampling
Rates TWA: STEL: |
0.05 to 0.15 L/min 0.3 L/min (15-min samples) | ||
| Recommended Maximum Air Volume: | 36 L (0.15 L/min for 240 min) | ||
| Analytical Procedure: | Sampling media is desorbed in 30% hydrogen peroxide and analyzed as phosphite by ion chromatography. | ||
| Detection
Limit Qualitative: Quantitative: |
0.009 ppm for a 36-L air sample 0.015 ppm for a 36-L air sample | ||
| Precision and
Accuracy Validation Range: CVT(pooled): Bias: Overall Error: |
| ||
| Method Classification: | Validated Method | ||
| Special
Requirements Collection Device: |
Certain lots or grades of beaded carbon have been inefficient in collecting phosphine. See Section 5 for information. | ||
| Sampling: | If sampling in low relative humidity (<40% RH)
areas, an | ||
| Analysis: | Analyze samples within 12 days after collection. Samples should be refrigerated to increase stability. | ||
| Chemist: Date (Date Revised): |
James Ku March, 1988 (June, 1991) | ||
descriptive use only and do not constitute endorsements by USDOL-OSHA.
Similar products from other sources can be substituted.
OSHA Technical Center
Salt Lake City, Utah
1. Introduction
This method describes the breathing zone sampling and laboratory analysis of workplace personnel for occupational exposure to phosphine. Collected samples are analyzed by ion chromatography.
- Prepare a KOH solution by adding 0.5 g KOH to 75 mL deionized
water (
DI H2O ).
- Using a hood for ventilation, carefully add the KOH solution
to 50 g of beaded activated carbon. Occasionally stir and then
allow the slurry to sit for a few minutes.
- Dry the impregnated carbon at 100°C for at least 2 h in a
drying oven.
- Cool the impregnated carbon to room temperature.
- Pack approximately 1.5 g of solid sorbent into 9-mm o.d.
(standard wall) × 12-cm Pyrex sampling tubes and hold in place
with glass wool plugs. Cap tubes with end caps or fire seal.
- Sampling tubes prepared in this fashion are stable for at least 6 months (8.9.).
- Insert a cellulose filter plug (Rainin Instrument Co., Woburn,
MA; part no. 23534/B) into each glass tube (9-mm o.d. × 10-cm
length).
- Cap the pre-tubes with end caps or fire seal.
- The plug is saturated with 0.75 mL
DI H2O before sampling. One humidifier tube provides enough water vapor for one sampling tube taken at the prescribed flow rate and time. - For humidification using an impinger, connect the impinger
(containing only 5 mL
DI H2O ) the sampling tube and then the pump with a minimum amount of flexible tubing. Sampled air must enter the impinger first and then the sampling tube.
- For humidification using a filter plug, use the pre-tube
assembled in Section 5.1.5.
(If a commercially prepared pre-tube is used, do not add any
water. The plug has already been moistened). Using an eyedropper
or pipette, add 0.75 mL
DI H2O to the filter plug. Connect the pre-tube in front of the solid sorbent tube with a minimum amount of tubing. Connect both tubes to the pump such that sampled air enters the pre-tube first.
- 1.1. History
- 1.1.1. Previously, a solid sorbent tube
containing silica gel impregnated with mercuric cyanide was used for
phosphine monitoring (8.1.).
After sample collection, a hot, acidic permanganate solution was
used for sample extraction and oxidation to phosphate. An organic
solvent extraction was also used and the phosphate was analyzed
calorimetrically as a phosphomolybdate complex. This analytical
method involved several time-consuming analytical steps and solution
transfers. The sampling material, mercuric cyanide, is toxic and
sampling tubes have been difficult to obtain through commercial
sources.
1.1.2. Although it has been reported that phosphine can be collected in bubblers containing acidified permanganate solution (8.2.) or silver diethyldithiocarbamate (AgDDC) dissolved in pyridine (8.3.), the analytical methods are complex and sampling is inconvenient. Collection of phosphine in permanganate solution requires a sampling train of bubblers containing large amounts of solution. The permanganate solutions are analyzed using the calorimetric phosphomolybdate method mentioned in Section 1.1.1. The AgDDC method requires samples to be analyzed within 8 h of sampling.
For these reasons, it was desirable to develop a more acceptable sampling and analytical method for phosphine.
Phosphine is collected on a solid sorbent consisting of beaded, activated carbon impregnated with potassium hydroxide. The sample collection and analysis are based on the following proposed chemical reaction:
For every 3 moles of phosphine collected, 1 mole of phosphate (HPO42-) and 2 moles of phosphite (HPO32-) are produced; therefore, the conversion factor (3PH3/2HPO32-) is 0.6376. The collected phosphine is extracted from the treated carbon beads with 30% hydrogen peroxide and analyzed as phosphite by ion chromatography (IC).
1.3. Advantages and Disadvantages
- 1.3.1. This method has adequate sensitivity for determining
compliance with the 0.3 ppm time weighted average (TWA) OSHA
Permissible Exposure Limit (PEL) for phosphine. The method is also
adequate for Short-Term Exposure Limit monitoring.
1.3.2. The samples are analyzed by means of a quick instrumental method that may be easily automated and computerized.
1.3.3. The method is specific for phosphine (determined as phosphite) in the presence of phosphate and other compounds.
1.3.4. The sampling device is portable and does not contain any highly toxic materials.
1.3.5. Desorption and preparation of samples for analysis involve simple procedures and equipment.
1.3.6. One disadvantage is an unacceptable decrease in recovery when sampling at a RH <40%. When sampling with an in-line humidifier, the recovery approaches that seen at higher humidities. The humidifier can be used regardless of the humidity level at the sampling site.
1.3.7. Another disadvantage is sample storage stability. Collected samples stored at 20 to 25°C for periods greater than 12 days gave unacceptably low recoveries. Samples should be analyzed within 12 days after sampling. Sample refrigeration improves stability, and is strongly recommended.
1.4. Phosphine (CAS 7803-51-2) uses (8.4.):
| Organic preparations | Phosphonium halides |
| Polymerization initiator | Condensation catalyst |
| Doping agent for solid state electronic components | |
Phosphine is also used as a fumigant insecticide or rodenticide. A metal phosphide (usually magnesium, calcium, zinc or aluminum) is used for fumigation. Phosphine is produced from the decomposition reaction of the phosphide with moisture in the ambient air.
1.5. Physical and chemical properties (8.4., 8.5.):
Phosphine is a colorless gas; impurities can give the gas a disagreeable, garlic-like odor. Some of the physical properties are:
| Solubility | Soluble in alcohol, ether and cuprous chloride solution; slightly soluble in cold water; insoluble in hot water. |
| Specific gravity | 1.185 |
| Melting point | -133.5°C |
| Boiling point | -85°C |
| Autoignition temp. | 37°C (pure gas) |
1.6. Toxicology
| Note: | Information listed within this section is a synopsis of current knowledge of the physiological effects of phosphine and is not intended to be used as a basis for OSHA policy. |
- 1.6.1. The main occupational route of exposure is inhalation of
phosphine vapor.
1.6.2. Phosphine is a central nervous system depressant and airway irritant. Symptoms of mild, non-chronic phosphine exposures are (8.6., 8.7.):
| weakness | fatigue | vomiting |
| vertigo | nausea | diarrhea |
| headache | respiratory irritation | |
| abdominal pain | ||
Symptoms may mimic a viral respiratory tract infection.
1.6.3. At higher concentrations, symptoms may be present as tremors, disturbances of gait, convulsions, and coma. Death from extreme phosphine exposure is usually attributed to pulmonary edema and, to a lesser extent, cardiac arrest.
1.6.4. The mechanism of phosphine toxicity is not well understood. The physiologic effects are thought to occur as a result of damage to enzyme systems (8.8.).
2. Range, Detection Limit and Sensitivity (8.9.)
- 2.1. This method was validated over the range of 0.15 to 0.67 ppm
using flow rates from 0.11 to 0.15 L/min and 240- to 360-min sampling
times.
2.2. The qualitative and quantitative detection limits of the method are 0.009 and 0.015 ppm phosphine (36-L air volume). Both detection limits are based on a 5-mL sample volume, a 50-µL injection volume, and a 3 microsiemens full scale output setting.
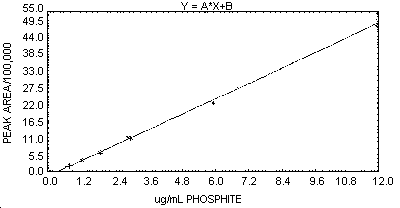
2.3. The sensitivity of the analytical method, when using the
instrumentation specified in Section 6.2.,
was calculated from the slope of a linear working range curve (0.61 to
12 µg/mL phosphate). The sensitivity for this curve was 433,020 area
units per 1 µg/mL phosphite (for the HP 3357 data reduction system
used, 1 area unit = 0.25 microvolt-second). A graphic representation
of a typical
3. Method Performance (8.9.)
- 3.1. The coefficient of variation for the overall sampling and
analytical method [CVT(pooled)] in the range
of 0.15 to 0.67 ppm was 0.041. Bias was +0.1% and overall error was
±8.3%. For the STEL (1.3 ppm was used) determinations, the
CV2 was 0.039, bias was -6% and overall
error was ±13.8%.
3.2. The collection efficiency for in-house and commercially (SKC Inc., Eighty Four, PA) prepared tubes was 100.0%. The parameters used for determining collection efficiency were: 50% RH, 0.12 to 0.15 L/min pump flow rate, 210- and 240-min sampling times and concentrations of 0.6 to 0.67 ppm.
3.3 There was no evidence of breakthrough after sampling 0.6 ppm phosphine for 360 min (50% RH and 25°C). A pump flow rate of 0.14 L/min was used for sampling. Sampling at a higher concentration (1.9 ppm) for 240 min gave an breakthrough value of less than 5%.
Breakthrough did occur after sampling at 30% RH without an in-line humidifier. Sampling was performed at a 0.115- to 0.135-L/min flow rate, 90-min sampling time, and a generation concentration of 1 ppm. The sample load at breakthrough was approximately 6 µg.
3.4. In storage stability studies, the mean recovery of samples stored at 20 to 25°C was 81% after 12 days, 64% after 18 days and 48% of the known concentration after 32 days. Samples refrigerated up to 30 days showed an acceptable improvement in recovery.
4. Interferences
- 4.1. When other compounds are known or suspected to be present in
the sampled air, such information should be transmitted with the
sample.
4.2. Any compound that has the same retention time as phosphite is an interference.
4.3. Interferences may be minimized by changing the operating conditions of the ion chromatography (e.g., changing the concentration of eluent and pump flow rate).
4.4. Water soluble phosphite salts will cause a positive interference; however, the collection of any particulate in a solid sorbent sampling tube should be minimal. If necessary, a pre-filter can be used to capture particulate.
- 5.1. Equipment
Sampling and pre-moistened humidifier tubes are commercially available [three companies offer sampling tubes: Forest Biomedical (Salt Lake City, UT), SKC Inc. (Eighty Four, PA), or Supelco (Bellefonte, PA)]. Two different size sampling tubes are available; a thin tube approximately 5-mm o.d. × 17-cm long and a wider tube approximately 9-mm o.d. × 11-cm long. Both tubes contain 1.5 g of treated sorbent. The 9-mm o.d. tube has similar dimensions as the humidifier tube. If a humidifier tube is not necessary, the thin (5-mm o.d.) tube can be used for added convenience. Sampling and humidifier tubes can also be prepared according to procedures listed in Sections 5.1.3.-5.1.5.
- 5.1.1. A personal sampling pump capable of sampling within ±5%
of the recommended flow rate of 0.05 to 0.15 L/min.
5.1.2. Tygon or other flexible tubing.
5.1.3. Carbon bead (Kureha Chemical Industry Co., 420 Lexington Ave., Suite 1742, NY, 10170, phone no. 212-867-7040).
Certain lots or grades of carbon bead (after treatment) have shown losses of up to 50% when sampling phosphine. The ability of the carbon bead to capture phosphine may be dependent on the pitch used to produce the bead and should be tested on a lot-by-lot basis. See reference 8.9. for an evaluation of carbon bead, Grade MU-AZ, lot 820601 or 15161.
5.1.4. Solid sorbent tubes are prepared by using beaded activated carbon. The beaded activated carbon is impregnated according to the following procedure:
| CAUTION: | Potassium hydroxide (KOH) can cause skin and eye irritation. Extended contact can cause serious burns. Avoid physical contact with this reagent. |
Approximately 30 tubes can be prepared from this recipe.
5.1.5. For low humidity (<40% RH) sampling, an in-line impinger or humidifier pre-tube is required. Humidifying pre-tubes are prepared by the following procedure:
5.2. Sampling Procedure
- 5.2.1. Measure the RH of the area to be sampled.
| Note: | Humidity measurements are not necessary if an in-line humidifier is used for each sample taken. The humidifiers can be used in high humidity environments. |
A battery-operated or sling psychrometer or a water vapor
detector tube can be used. If measurements indicate less than 40%
RH, or is expected to drop below 40% during sampling, an in-line
impinger containing approximately 5 mL of
5.2.2. If the RH is greater than or equal to 40%, a humidification device can still be used but is not required.
5.2.3. Place the sampling tube, impinger, or pre-tube and pump in appropriate positions on the employee.
5.2.4. For TWA determinations, sample at a flow rate of 0.15 L/min. Sample for a period up to 240-min per tube. For STEL measurements, sample at 0.3 L/min for 15 min.
5.2.5. Prepare a blank sample by treating a sampling tube in the same manner as the other sorbent tubes except that no air is drawn through it.
5.2.6. Cap and seal samples with OSHA Form 21 or other appropriate seals. Submit samples to the laboratory along with air volume and potential interference information. Request phosphine analysis.
5.2.7. All samples should be sent to the laboratory as soon as possible. If a delay in sample submission is unavoidable, refrigerate samples until shipment.
5.2.8. If bulk samples are also submitted, check hazardous substance shipping requirements and send in a separate shipping container.
5.2.9. Refrigerate these samples at the laboratory until analysis.
6. Analysis
Samples should be analyzed within 12 days of sampling. Refrigerated samples should be warmed to room temperature before preparation.
- 6.1. Precautions
- 6.1.1. Laboratory safety rules and regulations regarding
solution preparation and instrument operation must be followed.
6.1.2. Use gloves, lab coat, protective eyewear and an exhaust hood when handling hydrogen peroxide or sulfuric acid solutions.
6.1.3. Refer to the appropriate manuals for proper instrument operation and maintenance (8.10.).
- 6.2.1. Ion chromatography (model no. 2010i or 4500, Dionex,
Sunnyvale, CA) equipped with a conductivity detector.
6.2.2. Automatic sampler (model no. AS-1, Dionex) and 0.5 mL sample vials.
6.2.3. Laboratory automation system: Ion chromatography interfaced to a data reduction and control system (AutoIon 450, Dionex).
6.2.4. Micromembrane suppressor (model no. AMMS-1, Dionex).
6.2.5. Anion separator column (model no. HPIC-AS4A, Dionex) with pre-column (model no. HPIC-AG4A, Dionex).
6.2.6. Disposable syringes (1 mL).
6.2.7. Syringe pre-filters, 0.5 pm pore size (part no. SLSR 025 NS Millipore Corp., Bedford, MA).
| (Note: | Some syringe pre-filters are not cation- or anion-free. Tests should be done with blank solutions first to determine suitability for the analyte being determined). |
6.2.8. Assorted volumetric glassware: Micropipettes, volumetric flasks, graduated cylinders, beakers, and pipettes.
6.2.9. Analytical balance (0.01 mg).
Sodium carbonate (Na2CO3)
Sodium bicarbonate (NaHCO3)
Sodium phosphite (NaH2PO3 or Na2HPO3·5H2O)
Sodium phosphate (Na2HPO4)
- 6.3.1. Deionized water (
6.3.2. Eluent (0.002 M Na2CO3 + 0.001 M NaHCO3):
Dissolve 0.848 g Na2CO3 and 0.336 gram NaHCO3 in 4.0 L DI H2O.
6.3.3. Sulfuric acid (H2SO4),
6.3.4. Suppressor regenerant solution (0.02 N H2SO4):
Place 1.14 mL concentrated
H2SO4 into a 2-L
volumetric flask which contains approximately 500 mL
6.3.5. Standard stock solution, 1,000 µg/mL phosphite (as HPO32-):
Place 1.3000 g
NaH2PO3 (sodium
phosphite, monobasic) or 2.7000 g
Na2HPO3·5H2O
(sodium phosphite pentahydrate, dibasic) in a 1-L volumetric flask.
Add about 500 mL
6.3.6. Hydrogen peroxide, 30% (CAUTION: Solutions of 30% hydrogen peroxide can cause irritation or burns).
6.3.7. Phosphite standard solutions, 100, 10, and 1 µg/mL:
Pipette appropriate volumes of 1,000 µg/mL phosphite stock solution into volumetric flasks and dilute to the mark with eluent. Prepare monthly.
6.3.8. Standard stock solution 1,000 µg/mL phosphate (HPO42-):
Dissolve 1.4950 g
Na2HPO4 and
dilute to 1 L with
| Note: | The phosphate stock standard is only prepared as a source for preparing the mixed standard in Section 6.3.9. This mixed standard is used during the analysis to assure separation of the phosphite and phosphate peaks. |
6.3.9. Phosphate and phosphite mixed-standard solution: Make serial dilutions of the phosphite and phosphate stock solutions to achieve concentrations of about 5 to 10 µg/mL of both analytes. Prepare this mixture in eluent.
6.4. Sample Preparation
- 6.4.1. Carefully transfer the beaded activated carbon from each
sample tube into separate 25-mL Erlenmeyer flasks.
6.4.2. Using a hood for ventilation, carefully pipette 5 mL of 30% hydrogen peroxide into each flask and wait until the reaction stops (approximately 10 min). Occasionally stir the solution while allowing to cool to ambient temperature. Cap the flasks tightly and allow the solution to sit for at least 1 h.
6.4.3. Pipette a 0.5- to 0.6-mL portion of each sample solution into separate automatic sampler vials. Place a 0.5-mL filter cap into each vial. The large filter portion of the cap should face the sample solution.
6.5. Working Standard Preparation
- 6.5.1. Phosphite working standards may be prepared in the ranges
specified below:
| Working
Std µg/mL |
Standard
Solution µg/mL |
Aliquot mL |
| 0.5 | 1 | 5 |
| 1 | 1 | * |
| 3 | 10 | 3 |
| 6 | 10 | 6 |
| 10 | 10 | * |
| * Already prepared in Section 6.3.7. | ||
6.5.2. Pipette appropriate aliquots of standard solutions (prepared in Section 6.3.7.) into 10-mL volumetric flasks and dilute to volume with eluent. Prepare working standards weekly.
6.5.3. Pipette a 0.5- to 0.6-mL portion of each standard solution into separate automatic sampler vials. Also prepare a vial containing a mixed-standard solution (Section 6.3.9.). Place a 0.5-mL filter cap into each vial. The large filter portion of the cap should face the standard solution.
6.5.4. Load the automatic sampler with labeled samples and standards.
6.6. Analytical Procedure
- 6.6.1. Set up the ion chromatography in accordance with the
Standard Operating Procedure (8.10.).
Typical operating conditions using the equipment described in
Section 6.2.
are:
| Ion Chromatograph | |
| Eluent: | 0.002 M Na2CO3 + 0.001 M NaHCO3 |
| Columns: | separator column (HPIC-AS4A) with precolumn A(HPIC-AG4A) |
| Suppressor: | anion micromembrane (AMMS-1) |
| Column temperature: | ambient |
| Sample injection volume: | 50 µL |
| Pump | |
| Pump flow rate: | 2 mL/min |
| Pump pressure: | approximately 1000 psi |
| Chromatogram | |
| Run time: | 8 to 10 min |
| Retention time (HPO32-): | 4 to 5 min |
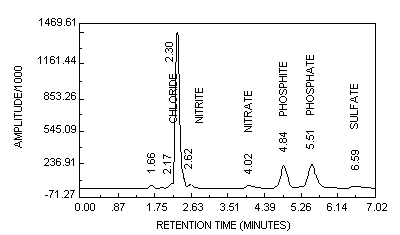
6.6.2. Determinations
Follow the Standard Operating Procedure (8.10.) for initiating the analysis. Always analyze a mixed phosphite/phosphate standard (Section 6.3.9.) to determine if sufficient resolution is present with the IC to separate phosphite and phosphate. A chromatogram for a 6 µg/mL (as phosphite) generated sample spiked with 12 µg/mL of phosphate is shown in Figure 2. After the analysis is completed, retrieve the computer-calculated sample and standard peak areas or heights.
7. Calculations
- 7.1. Prepare a concentration-response curve by plotting the
concentration of the standards in µg/mL (or µg/sample if the same
volumes are used for samples and standards) versus peak areas or peak
heights. An example of a typical curve constructed from peak areas is
shown in Figure 1.
7.2. The blank and sample concentrations are calculated from the regression equation. The calculated µg/mL phosphite blank value is then subtracted from the calculated sample values. If a different solution volume is used for samples and blank, subtract total µg blank values from total µg sample values.
7.3. The concentration of phosphine (PH3) in each air sample is expressed in ppm:
| ppm PH3 = | [24.45] [µg/mL Phosphite]
[sample volume (mL)] [Conversion]
[MW] [air volume (L)] |
Where:
| Molar volume | = | 24.45 (25°C and 760 mmHg) |
| µg/mL Phosphite | = | Calculated from curve (blank corrected) |
| Sample volume | = | 5 mL |
| Molecular weight (MW) of phosphine | = | 34.0 |
| [Conversion] of phosphite to phosphine | = | 0.6376 (from Section 1.2.) |
Therefore, for a 5 mL sample volume:
| ppm Phosphine = | [2.293] [µg/mL Phosphite]
air volume (L) |
7.4. Reporting Results
Report results to the industrial hygienist as ppm phosphine.
8. References
- 8.1. National Institute for Occupational
Safety and Health: NIOSH Manual of Analytical Methods. 2nd.
ed., Vol. 5 (Method No. S332) (DHEW/NIOSH Pub. No. 79-141).
Cincinnati, OH: National Institute for Occupational Safety and Health,
1979.
8.2. Barrett, W.J. and H.K. Dillon: Development of Methods for the Determination of Elemental Phosphorus and Phosphine in Air (DHEW Publication No. 78-177) Cincinnati, OH: National Institute for Occupational Safety and Health, 1978; p 52.
8.3. Dechant, R., G. Sanders, and R. Graul: Determination of phosphine in air. Am. Ind. Hyg. Assoc. J. 24: 164-167 (1963).
8.4. Hawley, G.G., ed.: The Condensed Chemical Dictionary. 9th ed. New York: Van Nostrand Reinhold Co., 1971.
8.5. Weast, R.C., ed.: CRC Handbook of Chemistry and Physics. 62nd ed. Boca Raton, FL: CRC Press, Inc., 1981.
8.6. Sittig, M.: Handbook of Toxic and Hazardous Chemicals. Park Ridge, NJ: Noyes Publications, 1981, pp 541-543.
8.7. Proctor, N.H. and J.P. Hughes: Chemical Hazards of the Workplace. Philadelphia, PA: J.B. Lippincott Company, 1978. pp 415-416.
8.8. Air Products and Chemicals Inc.: Specialty Gas Material Safety Data Sheet for Phosphine. Allentown, PA: Air Products and Chemicals Inc., 1984.
8.9. Occupational Safety and Health Administration Technical Center: Phosphine Backup Data Report (ID-180) by J.C. Ku. Salt Lake City, UT. Revised 1991.
8.10. Occupational Safety and Health Administration Technical Center: Standard Operating-Procedure-Ion Chromatography. Salt Lake City, UT. In progress (unpublished).