DETERMINATION OF CHLORINE DIOXIDE IN WORKPLACE ATMOSPHERES
| Method Number: | ID-202 |
| Matrix: | Air |
| OSHA Permissible Exposure
Limits Final Rule Limits: |
0.1 ppm Time Weighted Average (TWA) 0.3 ppm |
| Transitional Limit: | 0.1 ppm TWA |
| Collection Device: | An air sample is collected using a calibrated sampling pump and a midget fritted glass bubbler. The bubbler contains a collection solution of 0.02% potassium iodide (KI) in a sodium carbonate/sodium bicarbonate buffer. |
| Recommended Sampling Rate | 0.5 Liter per minute (L/min) |
| Recommended Air
Volume TWA: STEL: |
120 L (0.5 L/min for 240 min) 7.5 L (0.5 L/min for 15 min) |
| Analytical Procedure: | In the weakly basic solution, chlorine dioxide reacts with KI to form chlorite (ClO2-) which is then determined by an ion chromatograph equipped with a conductivity detector and gradient pump. |
| Detection Limit Qualitative: |
0.001 ppm (120-L air sample) 0.018 ppm (7.5-L air sample) |
| Quantitative: | 0.004 ppm (120-L air sample) 0.059 ppm (7.5-L air sample) |
| Precision and Accuracy Validation Range: |
0.058 to 0.202 ppm |
| CVT: | 0.076 |
| Bias*: | +0.05 |
| Overall Error*: | ±20% |
| Method Classification: | Validated Method |
| Chemist: | James C. Ku |
| Date (Date Revised): | June, 1990 (Feb., 1991) |
* As compared to the NIOSH chlorine dioxide method (chlorophenol red)
descriptive use only and do not constitute endorsements by USDOL-OSHA.
Similar products from other sources can be substituted.
OSHA Technical Center
Salt Lake City, Utah
1. Introduction
This method describes the sample collection and analysis of airborne chlorine dioxide (ClO2). Samples are taken in the breathing zone of workplace personnel, and analysis is performed by ion chromatography (IC).
- Chlorine produces a significant positive interference;
- The stock solution used for ClO2 analysis is very difficult to prepare and extremely unstable.
- Sodium bicarbonate (NaHCO3)
- Sodium carbonate
(Na2CO3)
Potassium iodide (KI)
- Sodium chloride (NaCl)
- Sulfuric acid
- Sodium thiosulfate solution (Na2S2O3), 0.1 N, traceable to a primary standard (Cat. No. SS368-1, Fisher Scientific, Pittsburgh, PA). Any expiration date must be adhered to. This solution can be prepared and standardized according to procedures in reference 8.12.
- Sulfuric acid (H2SO4), concentrated.
- Sulfuric acid, dilute.
Slowly and cautiously add 40 mL of concentrated H2SO4 to a 200-mL volumetric flask which contains 150 mL DI H2O. Allow to cool, then dilute to volume with DI H2O.
- Potassium iodide (KI).
- Starch indicator solution, (1% w/v): Gradually add about 5 mL of DI H2O to 1 g soluble starch, with stirring, until a paste is formed. Add the paste to 100 mL of boiling DI H2O. Allow to cool, then add 5 g KI and stir until the KI is dissolved. Prepare a fresh solution for each standardization. Alternatively, a commercial indicator can be used (Starch indicator, Cat. No. 8050, Ricca Chemical Co., Arlington, TX).
- Add 10 mL of dilute H2SO4 into a 125-mL Phillips beaker which contains 20.0 mL of NaClO2 stock solution (1,000 µg/mL, from Section 6.3.9.).
- Add 1 g of KI and 40 mL of DI H2O.
- Titrate with standardized 0.1 N Na2S2O3 until a color change to a light straw color is achieved.
- Add 2 mL of 1% starch indicator. A blue color should appear.
- Titrate again with 0.1 N Na2S2O3 until the blue color completely disappears.
- For blank sample(s), repeat steps 1 through 5 except use 20.0 Ml DI H2O instead of 20.0 mL of the NaClO2 stock solution.
- Calculate µg/mL chlorite as follows:
µg/mL ClO2- = (A - B)(C)(D)
EWhere: A = mL of the standardized Na2S2O3 solution required to titrate the sample B = mL of the standardized Na2S2O3 solution required to titrate the blank C = normality of the standardized Na2S2O3 solution (meq/mL) D = (16.875 mg/meq- ClO2-)(1,000 µg/mg) = 16.875 × 103 µg/meq of ClO2- E = mL of ClO2- used = 20 mL - Prepare chlorite (or chloride, or a chlorite and chloride
mixture) working standards in the ranges specified below:
Working std Standard Aliquot µg/mL
Solution µg/mL
mL
0.5 1 5 1 1 * 2 10 2 5 10 5 10 10 * 20 100 2 *Already prepared in Section 6.3. - Pipette appropriate aliquots of standard solutions (prepared
in Section 6.3.)
into
10-mL volumetric flasks and dilute to volume with collection solution.
- 1.1. History
The previous method used to determine
ClO2 in the workplace involved collecting
samples in 0.01 N sodium hydroxide (8.1.).
Because this method was also used to collect chlorine
(Cl2) and could not discriminate between the
two species, a better method was needed. The scientific literature
contains few articles addressing Cl2 and
ClO2 analysis. A method proposed by NIOSH
was a spectrophotometric technique based on the decolorization of
chlorophenol red (CPR) by ClO2 (8.2.).
Another method was proposed by the Workers' Compensation Board of
British Columbia as the
After reviewing and checking the CPR method, it was found that:
A comparison of the CPR and NNDP method indicated a disagreement in results below 0.3 ppm ClO2; NIOSH speculated this was due to shortcomings in the iodometric method (8.2.).
For the volumetric NNDP method, the analysis is a time-consuming process, which uses an unstable reagent (NNDP) for color development (8.4.). The method described herein uses a common analytical technique and is not susceptible to an interference from Cl2. During the evaluation of this method (1988), a paper was published in the literature which describes a similar sampling and analytical approach (8.5.); however, the collection solution the authors suggest using is buffered to a neutral instead of a weakly basic pH.
1.2. Principle
Chlorine dioxide is collected in a midget fritted glass bubbler (MFGB), containing 0.02% potassium iodide (KI) in a sodium carbonate/sodium bicarbonate (Na2CO3/NaHCO3) buffer solution. Chlorine dioxide as well as chlorine are trapped and converted to chlorite (ClO2-) and chloride (Cl-), respectively, in neutral or a weak basic solution according to the following chemical reactions:
The collected ClO2 (as ClO2-) is analyzed by IC using a conductivity detector. A gradient pump is used to facilitate the elution of the iodide ion present in the sampling solution. The amount of Cl2 collected can be estimated as Cl-; however, the evaluation of this method did not include a full validation of the sampling and analysis of Cl2. Therefore, results for Cl2 are only used as a screening tool. For further information regarding sampling and analysis of Cl2, see OSHA method no. ID-101.
1.3. Advantages and Disadvantages
- 1.3.1. This method has adequate sensitivity for determining
compliance with the OSHA
1.3.2. The method is simple, rapid, and easily automated.
1.3.3. The analysis is specific for ClO2 (determined as chlorite ion, ClO2-), in the presence of Cl2.
1.3.4. This method requires the use of a gradient pump during analysis in order to allow the iodide contained in the collection solution to elute and still have a reasonably short analysis time.
1.3.5. A disadvantage is the need to prepare standards from a ClO2- stock solution. This solution, prepared from technical-grade sodium chlorite (about 80% purity), is unstable and must be standardized monthly.
1.3.6. Another disadvantage is the sampling device. Use of impinger collection techniques may impose inconveniences. Spillage can occur during sampling, handling, and transportation to the laboratory.
1.4. Physical Properties (8.6., 8.7.)
Chlorine dioxide (CAS No. 10049-04-4):
| Chemical formula | ClO2 |
| Molecular weight | 67.5 |
| Specific gravity | 1.642 at 0°C (liquid) |
| Melting point | -59.5°C |
| Boiling point | 10°C |
| Vapor pressure | 96 KPa (720 mmHg) at 20°C |
| Vapor density | 3.09 g/L |
| Synonym | chlorine peroxide |
| Other characteristics | Highly toxic, strong oxidizing agent, soluble and decomposes in water, dissolves in alkalis forming a mixture of chlorite and chlorate. Explodes when exposed to light, heated, or by reaction with organic materials. |
1.5. Some sources for potential ClO2 exposures are (8.6.):
- Bleaching wood pulp, fats, oils, and flour
production
Removing tastes and odors from water supplies
Biocide
Disinfectant
Odor control
Flour maturing operations
Additive in swimming pools
1.6. Toxicology
Note: Information listed within this section is a synopsis of current knowledge of the physiological effects of ClO2 and is not intended to be used as the basis for OSHA policy.
Data from human exposures indicate that marked irritation occurs on inhalation of 5 ppm (no length of exposure specified), and that one death occurred at 19 ppm. Repeated exposures in humans have been linked to bronchitis and pronounced emphysema. Clinical studies revealed that the majority of workers who had been exposed for five years to average concentrations of ClO2 below 0.1 ppm, combined with about 1 ppm Cl2, experienced eye and respiratory irritation and slight bronchitis. Some gastrointestinal irritation was also observed in three workers (8.8.).
2. Range, Detection Limit, and Sensitivity (8.9.)
- 2.1. This method was validated over the concentration range of
0.058 to 0.202 ppm. An air volume of 120 L and a flow rate of 0.5
L/min were used. Samples were taken for 240 min.
2.2. The qualitative detection limit was 0.025 µg/mL or 0.375 µg (as ClO2-) when using a 15-mL solution volume. This corresponds to 0.001 ppm ClO2 for a 120-L air volume.
2.3. The quantitative detection limit was 0.082 µg/mL or 1.23 µg (as ClO2-) when using a 15-mL solution volume. This corresponds to 0.004 ppm ClO2 for a 120-L air volume. A 50-µL sample injection loop and a detector setting of 1 microsiemen (µS) were used for both detection limit determinations.
2.4. The sensitivity of the analytical method was calculated from the slope of a linear working range curve (0.5 to 10 µg/mL chlorite). The sensitivity for this curve was 4.07 × 106 area units per 1 µg/mL when using the instrumentation mentioned in Section 6.2.
3. Method Performance (8.9.)
- 3.1. This method was compared to the NIOSH chlorophenol method for
ClO2 (8.2.).
All results were obtained using the NIOSH reference method results as
known values. Bias and overall error values are reported below as
compared to the NIOSH method.
3.2. The pooled coefficient of variation (CVT), for samples taken at about 0.5, 1, and 2 times the TWA PEL (0.05 to 0.2 ppm) was 0.076. The method exhibited slight positive bias (+0.05) for this concentration range. The overall error was within acceptable limits (< ±25%) at ±20%.
3.3. The CV2(pooled) for samples taken in the range of 0.028 to 0.33 ppm (about 0.3 to 3 times the TWA PEL) was 0.072. The method exhibited a slight positive bias (+0.033) and overall error was acceptable at ±18% for this broader concentration range.
3.4. The collection efficiency at 0.2 ppm ClO2 was 100%. Samples were collected at a generated concentration of 0.202 ppm ClO2 for 240 min.
3.5. A breakthrough test was performed at a concentration of 0.33 ppm ClO2. No breakthrough was found for a sampling time of 240 min at an average sample flow rate of 0.5 L/min. Under the same conditions, for a concentration of 0.67 ppm, the average breakthrough of ClO2 into a second impinger was 9.1%. At a flow rate of 1 L/min, about 10% breakthrough occurred after 90 min at a concentration of approximately 0.35 ppm ClO2.
3.6. Samples can be stored at normal (20 to 25°C) laboratory conditions for at least 96 days. Results of samples analyzed after 96 days were still within ±10% of the mean of samples analyzed after one day of storage. Samples were stored unprotected from light on a laboratory bench.
4. Interferences
- 4.1. Any compound having the same retention time as chlorite, when
using the operating conditions described, is an interference.
4.2. Interferences may be minimized by changing the eluent concentration and/or pump flow rate, or by using concentration gradient techniques.
4.3. Contaminant anions normally found in the workplace, such as nitrate (NO3-), sulfate (SO42-), and phosphate (HPO42-), do not interfere. However, very large amounts (> 100 µg/mL) of Cl- may interfere with the determination of ClO2. The possibility of collecting this quantity of Cl- in the workplace is minimal.
Particulate chloride contamination will present a positive interference for the screening determination of Cl2. Care must be exercised to not contaminate the collection solutions with chloride salts if screening for Cl2 is desired.
4.4. When other compounds are known or suspected to be present in the air, such information should be transmitted with the sample.
4.5. Altering the pH of the collection solution to more acidic conditions will alter the reaction of ClO2 to ClO2-. If strongly acidic gases are present in the sampled atmosphere and convert the buffer to an acidic solution, the reaction will not proceed in the fashion mentioned in Section 1.2. The following reaction would most likely occur:
The collection solution should have adequate buffering capacity for most industrial hygiene monitoring situations; however, sampling times should be decreased to maintain slightly basic conditions if sampling in the presence of large concentrations of acid gases (i.e. sulfur dioxide). The pH of the solution can also be measured with pH paper after sampling to determine if the collection solution has become acidic. If acidic, discard the sample and resample using shorter sampling times.
5. Sampling
- 5.1. Equipment and Reagents
- 5.1.1. Calibrated personal sampling pumps - capable of sampling
within ±5% of the recommended flow rate of 0.5 L/min.
5.1.2. Midget fritted glass bubblers (MFGBs)
5.1.3. Shipping vials: Glass scintillation vials, 20 mL, with Teflon-lined caps.
5.1.4. A stopwatch and bubble tube or meter - for pump calibration. Place a calibration MFGB containing 10 to 15 mL of collection solution in-line during flow rate calibration of each pump.
5.1.5. Various lengths of polyvinyl chloride (PVC) tubing are used to connect the MFGBs to pumps.
5.1.6. Buffer solution (1.5 mM Na2CO3/1.5 mM NaHCO3):
Dissolve 0.636 g Na2CO3 and 0.504 g NaHCO3 in 4.0 L of deionized water.
5.1.7. Collection solution:
Dissolve 0.2 g KI in 1.0 L of buffer solution.
5.2. Sampling Procedure
- 5.2.1. Place 15 mL of collection solution in a MFGB, and then
connect the bubbler to a calibrated sampling pump using PVC tubing.
Position the MFGB in the breathing zone of the employee.
5.2.2. For STEL determinations, collect the sample at a flow rate of 0.5 L/min and a sampling time of at least 15 min. For TWA samples, an air volume of 120-L is recommended at 0.5 L/min. Take enough samples to cover the workshift being monitored.
5.2.3. After sampling, transfer the bubbler solution into a 20-mL glass scintillation vial. Rinse the bubbler with 2 to 3 mL of unused collection solution and transfer the rinsings into the sample vial. Place the Teflon-lined cap tightly on the vial and seal the cap with vinyl or waterproof tape to prevent leakage during shipment.
6. Analysis
- 6.1. Precautions
- 6.1.1. Refer to instrument and standard operating procedures
(SOP) for proper operation (8.10.,
8.11.).
6.1.2. Observe laboratory safety regulations and practices.
6.1.3. Sulfuric acid (H2SO4) can cause severe burns. Wear protective gloves, labcoat, and eyewear when using concentrated H2SO4.
6.2. Equipment
- 6.2.1. Ion chromatograph (Model 4000i or 4500i with a
concentration-gradient pump, Dionex, Sunnyvale, CA) equipped with a
conductivity detector.
6.2.2. Automatic sampler (Model AS-1, Dionex) and sample vials (0.5 mL).
6.2.3. Laboratory automation system: Ion chromatograph interfaced to a data reduction system.
6.2.4. Anion separator column with precolumn (Model HPIC-AS4A and AS4G, Dionex).
6.2.5. Anion suppressor (Model AMMS-1 micromembrane suppressor, Dionex).
6.2.6. Disposable syringes (1 mL) and filters.
(Note: Some syringe pre-filters are not cation- or anion-free. Tests should be done with blank solutions first to determine suitability for the analyte being determined).
6.2.7. Miscellaneous volumetric glassware: Micropipettes, buret, volumetric flasks, graduated cylinders, and beakers.
6.2.8. Analytical balance (0.01 mg).
6.3. Reagents - All chemicals should be at least reagent grade (Note: Sodium chlorite may only be commercially available as technical grade)
- 6.3.1. Eluent 1: Deionized water (DI
H2O) with a specific conductance of less
than 10 µS.
6.3.2. Eluent 2 (10 mM Na2CO3):
Dissolve 2.12 g Na2CO3 in 2.0 L of DI H2O.
6.3.3. Eluent 3 (10 mM NaHCO3):
Dissolve 1.68 g NaHCO3 in 2.0 L of DI H2O.
6.3.4. Buffer solution (1.5 mM Na2CO3/1.5 mM NaHCO3):
Dissolve 0.636 g Na2CO3 and 0.504 g NaHCO3 in 4.0 L of DI H2O.
6.3.5. Collection solution:
Dissolve 0.2 g KI in 1.0 L of buffer solution.
6.3.6. Regeneration solution (0.02 N H2SO4):
Place 1.14 mL concentrated H2SO4 into a 2-L volumetric flask which contains about 500 mL DI H2O. Dilute to volume with DI H2O.
6.3.7. Chloride stock standard (1,000 µg/mL):
Dissolve 1.6479 g dried NaCl and dilute to the mark in a 1-L volumetric flask with DI H2O.
6.3.8. Chloride standards (100, 10, and 1 µg/mL):
Perform serial dilutions of the 1,000 µg/mL chloride stock standard with collection solution. Prepare weekly. [Note: Prepare only if necessary. These standards are only used to screen Cl2 (as Cl-) concentrations.]
6.3.9. Chlorite stock standard (1,000 µg/mL):
Dissolve in a 1-L volumetric flask approximately 1.7 g sodium chlorite (NaClO2) in 500 mL DI H2O. Dilute to the mark with DI H2O. Wrap the volumetric flask with aluminum foil and store in a refrigerator at about 4°C. This solution must be standardized monthly as described in Section 6.4.1.
6.3.10. Chlorite standard (100 µg/mL). Dilute 10 mL of the 1,000 µg/mL chlorite stock standard to 100 mL with collection solution. Prepare monthly.
6.3.11. Chlorite standard (10 µg/mL). Dilute 10 mL of the 100 µg/mL chlorite stock standard to 100 mL with collection solution. Prepare weekly.
6.3.12. Chlorite standard (1 µg/mL). Dilute 10 mL of the 10 µg/mL chlorite stock standard to 100 mL with collection solution. Prepare weekly.
6.3.13. Reagents for standardizing the chlorite stock standard solution:
Note: If a 0.1 N (< ±0.5% variation) sodium thiosulfate solution traceable to a primary standard is unavailable, any laboratory-prepared sodium thiosulfate solutions must be standardized according to procedures listed in reference 8.12. Standardize any sodium thiosulfate solution has aged significantly.
6.4. Standard Preparation
- 6.4.1. Standardization of chlorite stock
solution (Note: This procedure is adapted from those found in
reference 8.12.)
6.4.2. Working standard preparation:
6.4.3. Pipette a 0.5- to 0.6-mL portion of each standard solution into separate automatic sampler vials. Place a 0.5-mL filter cap into each vial. The large exposed filter portion of the cap should face the standard solution.
6.4.4. Prepare a reagent blank from the collection solution.
6.5. Sample Preparation
- 6.5.1. Carefully transfer sample solutions
from the 20-mL glass scintillation vials into
6.5.2. If the sample solutions contain particulate, remove the particles using a pre-filter and syringe. Fill the 0.5-mL automatic sampler vials with sample solutions and push a 0.5-mL filtercap into each vial.
6.5.3. Load the automatic sampler with labeled samples, standards and blanks.
6.6. Analytical Procedure
- 6.6.1. Set up the ion chromatograph in accordance with the SOP
(8.10.).
Typical operating conditions for a Dionex 4000i or 4500i with an automated sampler are listed below.
| Gradient pump | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eluent 1: | DI H2O | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eluent 2: | 10.0 mM Na2CO3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eluent 3: | 10.0 mM NaHCO3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pump pressure: | approximately 900 psi | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Flow rate: | 2 mL/min | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||
| * | Gradient change in eluent concentration from 3.1 to 11.1 min is performed to facilitate elution of iodide present in the collection solution. |
| ** | Gradient return in eluent concentration to initial analytical conditions. |
| Column & Sample Injection | |
| Column: | HPIC-AS4A |
| Column temperature: | ambient |
| Sample injection loop: | 50 µL |
| Chromatogram | |
| Run time: | 18 min |
| Peak retention time: | |
| ClO2- | approximately 2 min |
| Cl- | approximately 3 min |
6.6.2. Follow the SOP (8.10.) for further analytical instructions.
7. Calculations
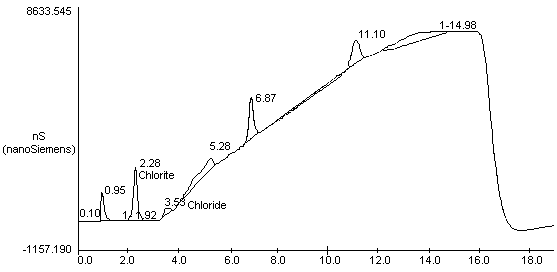
- 7.1. After the analysis is completed, the peak areas and heights
can be retrieved using a variety of methods or programs. Obtain hard
copies of chromatograms from a printer. A chromatogram of a mixed
standard of 5 µg/mL ClO2- and 0.5
µg/mL Cl- is shown in Figure 1.
7.2. Prepare a concentration-response curve by plotting the concentration of the standards in µg/mL versus peak areas or peak heights. Determine the concentration (µg/mL) of each sample by comparing the area or height to the curve. Blank correct all samples as shown:
| Where: | ||
| µgC Analyte | = | Corrected amount (µg) in the sample solution. |
| S | = | µg/mL sample (from curve) |
| SV | = | Sample solution volume, mL (from Section 6.5.1.) |
| BL | = | µg/mL blank (from curve) |
| BLV | = | Blank solution volume, mL (from Section 6.5.1.) |
7.3. The concentration of ClO2 and Cl2 in each air sample is expressed in ppm.
| ppm ClO2 = | µgC analyte × molar volume
air volume × molecular weight |
| ppm Cl2* = | µgC analyte × molar volume
air volume × molecular weight |
| Where: | ||
| µg/mL ClO2- or Cl- | = | Amount found from the curve |
| Solution volume (mL) | = | Amount of sample (from Section 6.5.1.) |
| Molar volume | = | 24.45 (25°C and 760 mmHg) |
| Molecular weight for ClO2 | = | 67.5 |
| Molecular weight for Cl2 | = | 71.0 |
* Note: Results for Cl2 are used for screening purposes only.
7.4. Reporting Results
Report results to the industrial hygienist as ppm chlorine dioxide. Results determined for exposure to chorine may be used as information to the industrial hygienist. Additional sampling for chlorine may be recommended using OSHA method no. ID-101.
8. References
- 8.1. Occupational Safety and Health
Administration Analytical Laboratory: Chlorine Dioxide
(Tentative), Internal Document. Salt Lake City, UT, 1971
(unpublished).
8.2. National Institute for Occupational Safety and Health: Methods Development for Sampling and Analysis of Chlorine, Chlorine Dioxide, Bromine, and Iodine - Research Report for Chlorine Dioxide by W.K. Fowler and H.K. Dillon. Birmingham, AL: Southern Research Institute (Contract no. 210-80-0067), 1982.
8.3. Laboratory Services, Worker's Compensation Board of British Columbia: Chlorine Dioxide in Air (Analytical Method No. 0350). Vancouver, B.C., Canada: Worker's Compensation Board of British Columbia, Draft Copy, 1982.
8.4. National Council of the Paper Industry
for Air and Stream Improvement, Inc.: A Laboratory
Investigation of an Iodometric Method for Determining Chlorine and
Chlorine Dioxide in Pulp and Paper Industry Workplace Atmospheres
(Technical Bulletin No. 409). New York: NCASI, September 1983.
Also: Development and Laboratory Evaluation of Improved
Iodometric Methods for Determining Chlorine and Chlorine Dioxide in
Pulp and Paper Industry Workplace Atmospheres (Technical Bulletin
No. 521). April, 1987.
8.5. Bjorkholm, E., A. Hultman, and J. Rudling: Determination of chlorine and chlorine dioxide in workplace air by impinger collection and ion-chromatographic analysis. J Chromatogr. 457: 409-414 (1988).
8.6. Hawley, G.G.: The Condensed Chemical Dictionary. 11th ed. New York: Van Nostrand Reinhold Co., 1987.
8.7. Weast, R.C., ed.: CRC Handbook of Chemistry and Physics. 59th ed. Boca Raton, FL: CRC Press, Inc., 1979.
8.8. "Chlorine Dioxide" Federal Register 54:12 (19 Jan. 1989). p. 2508.
8.9. Occupational Safety and Health Administration Technical Center: Chlorine Dioxide Backup Data Report (ID-202). Salt Lake City, UT. Revised 1991.
8.10. Dionex Corporation: 4000i and 4500i Ion Chromatograph Operation and Maintenance Manual. Sunnyvale, CA: Dionex Corporation, 1988.
8.11. Occupational Safety and Health Administration Technical Center: Ion Chromatography Standard Operating Procedure. Salt Lake City, UT. In progress (unpublished).
8.12. American Society for Testing and Materials: Standard Recommended Practices for Apparatus, Reagents, and Safety Precautions for Chemical Analysis of Metals (Annual Book of ASTM Standards, Part 12, E-50). Philadelphia, PA: American Society for Testing and Materials, 1978.
| PEAK NUM |
RET TIME |
PEAK NAME |
AREA |
HEIGHT |
|
| ||||
| 2 3 4 5 6 7 8 9 10 |
0.95 1.47 1.92 2.28 3.53 5.28 6.87 11.10 14.78 |
chlorine chlorine |
1.028e+007 1.696e+005 1.482e+005 2.222e+007 4.259e+006 2.713e+007 1.569e+007 6.284e+006 3.672e+007 |
1179972 18079 19795 2174692 343291 428639 1678083 960400 6410 |