ETHYLENE DIBROMIDE
| Method no.: | 02 | |
| Matrix: | Air | |
| OSHA PEL: | 154 mg/m3 (20 ppm) | |
| Target concentration: | 1 mg/m3 (0.13 ppm) | |
| Procedure: | Collection on charcoal adsorbent, desorption with xylene, analysis by gas chromatography using an electron capture detector. Samples should be refrigerated and analyzed as soon as received. (Read attached revision.) | |
| Detection limit based on recommended air volume: (For analytical procedure only) |
0.005 mg/m3 (6.5 × 10-4ppm) | |
| Recommended air volume and sampling rate: |
10 L at 0.2 L/min | |
| Standard error of estimate at the target concentration: (Figure 4.5.2.): |
9.3% | |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. | |
| Date: April 1979 November 1981 Revision Attached |
Chemist: Dee R. Chambers Chemist: Carl J. Elskamp | |
Organic Methods Evaluation Branch
OSHA Analytical
Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
In 1953, the American Conference of Government Industrial
Hygienists adopted a TLV of 25 ppm as an
OSHA has adopted a 20 ppm TWA and a 30 ppm ceiling for EDB. (Ref. 5.2.) In July 1975, NIOSH issued a "Current Intelligence Bulletin" indicating that EDB has caused cancer in laboratory animals. For this reason, we anticipate that the present PEL may be lowered. This necessitates that the present analytical and sampling procedures be evaluated at a lower level to assure that an adequate method exists. The OSHA Analytical Laboratory analyzed 28 EDB samples during 1978.
1.1.2. Toxic effects (This section is for information only and should not be taken as basis of OSHA policy.)
Human: Direct contact with EDB causes irritation and injury to the skin and eyes. Exposure to the vapor has caused the development of respiratory tract inflammation along with anorexia and headache with recovery after discontinuance of exposure. Weakness and rapid pulse have been associated with EDB exposure as well as cardiac failure leading to death.
Oral ingestion of EDB has led to liver necrosis and kidney tubular damage. Other symptoms which may be encountered following ingestion include excitement, tinnitus, and severe protracted vomiting. (Ref. 5.3.)
In April 1978, NIOSH issued a recommendation that no worker be exposed to both EDB and disulfiram. This recommendation was based on research suggesting a serious toxic interaction between inhaled EDB and ingested disulfiram. Disulfiram is used as an alcohol deterrent and it is possible that 100,000 people may be ingesting disulfiram as therapy. (Ref. 5.4.)
Animal: In laboratory rodents, the vapor of EDB has caused renal and hepatic damage, depression of the central nervous system, and pulmonary irritation. Oral administration to hens adversely influenced the production, size and fertility of eggs. The semen from bulls given oral doses of EDB had low density and the spermatozoa had poor motility.
The National Cancer Institute tested EDB in rats and mice and found a 76 to 87% occurrence of squamous cell carcinoma of the stomach. No stomach tumors were observed among untreated rats and mice. (Ref. 5.3.)
1.1.3. Workplace exposure
NIOSH estimates that 9100 workers are exposed to EDB in industry. This does not include the 650,000 persons employed in service stations where leaded gasoline is used.
About 300 million pounds of EDB are produced annually in this country. Approximately 50% of this is used in fuel additives. EDB reacts with lead in gasoline, aiding the removal of lead from the engine cylinder. Much of the remaining 50% is used in fumigants. Approximately 98% of the 9100 potentially exposed workers are in the fumigation and extermination industry. (Ref. 5.3.)
1.1.4. Physical properties
EDB is a colorless, nonflammable liquid with a sweetish odor. Its odor is detectable at 10 ppm. It is emulsifiable. It is miscible with most solvents and thinners and slightly soluble in water. The specific gravity is 2.17 (20°C). Its vapor pressure is 17.4 mm Hg (30°C) and its boiling point is 131°C. It has no flash point. (Ref. 5.5.) The molecular weight is 188.
1.2. Detection limit, precision, sensitivity, and working range
- 1.2.1. The detection limit for the analytical procedure is 54 pg
per injection with a coefficient of variation of 0.23. (Section
4.1.) The detection limit was determined using
1.2.2. The pooled coefficient of variation of the analytical procedure over the range of 1.25 to 5 µg per sample is 0.045. (Section 4.2.) This represents an air concentration range of 0.125 to 0.5 mg/m3 based on the recommended sampling volume of 10 L.
1.2.3. The sensitivity of the analytical procedure over the
concentration range of 0.125 to 0.5 mg/m3
based on a
1.2.4. The estimated working range extends from the detection limit to the limit set by the capacity of the charcoal, provided the desorption efficiency is acceptable (>75%) at all concentrations.
1.3. Accuracy
- 1.3.1. The overall procedure must provide results that are
within 25% of the true value or better at the 95% confidence
interval.
1.3.2. The recovery of analyte from the charcoal tube after storage must be 75% or greater.
1.3.3. The recovery of samples stored at ambient temperatures dropped below 75%. Therefore, it is necessary to keep the samples refrigerated and to analyze them as soon as they are received. The overall accuracy and stability curve is shown in Section 4.5.
1.4. Advantages
- 1.4.1. The method of sampling is convenient. Charcoal tubes are
easily transported to the laboratory through the mail. The
analytical method is quick. Automation of the procedure is possible.
1.4.2. The electron capture detector, while being very sensitive to EDB, is not very sensitive to many hydrocarbons, thereby making the method more selective for EDB than the previous flame ionization method.
1.5. Disadvantages
It may be difficult to analyze for additional components on the same charcoal tube because of the solvent and detector used in this method.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. An approved and calibrated personal sampling pump whose
flow can be determined within ±5% at the recommended flow.
2.1.2. Charcoal tubes: Glass tube, with both ends heat sealed,
7.0 cm × 6-mm o.d. ×
2.2. Reagents
None required.
2.3. Sampling technique
- 2.3.1. Immediately before sampling, break off the ends of the
charcoal tube. All tubes must be from the same lot.
2.3.2. Connect the charcoal tube to the sampling pump with flexible tubing. The short section of the charcoal tube is used as a backup and should be positioned nearer the sampling pump.
2.3.3. The tube should be placed in a vertical position during sampling to minimize channeling.
2.3.4. Air being sampled should not pass through any hose or tubing before entering the charcoal tube.
2.3.5. Seal the charcoal tube with plastic caps immediately after sampling. Also, seal each sample lengthwise with an OSHA sealing tape.
2.3.6. With each batch of samples, submit at least one blank tube from the same lot used for samples. This tube should be subjected to exactly the same handling as the samples (break, seal, transport) except that no air is drawn through it.
2.3.7. Transport the samples (and corresponding paperwork) to the laboratory for analysis.
2.3.8. If bulk samples are submitted for analysis, they should be
transported in glass containers with
2.4. Breakthrough
Two samples were drawn from an atmosphere containing 2.2 mg/m3 EDB and 80% relative humidity. The air sampled for one sample was 12 L at 0.2 L/min and the other was 18 L at 0.2 L/min. The front and backup portions were each desorbed with 1 mL of xylene. Neither backup portion contained any EDB.
2.5. Desorption efficiency
- 2.5.1. Desorption tubes, 6 each, SKC Lot 107 charcoal, were
spiked with 2.5, 5, and 10 L of a solution containing 1 L/mL EDB in
xylene. This represents concentrations of 0.55, 1.1, and 2.2
mg/m3 based on a
2.5.2. The desorption efficiency may vary from laboratory to laboratory and for each lot of charcoal.
2.6. Recommended air volume and sample rate
- 2.6.1. The recommended air volume is 10 L.
2.6.2. The recommended sampling rate is 0.2 L/min.
2.7. Interferences
- 2.7.1. At the present time, it is unknown if any compound would
severely interfere with the collection of EDB on charcoal. In
general, the presence of other solvents will decrease the
breakthrough volume for a particular solvent.
2.7.2. Any compound which is suspected of interfering in the collection or analysis should be listed on the sampling data sheet.
2.8. Safety precautions
- 2.8.1. Safety glasses should be worn when breaking the ends of
the charcoal tubes.
2.8.2. Do not provide any spark source from pumps or other equipment when working in environments containing flammable vapors.
2.8.3. Observe all usual safety practices when sampling in hazardous areas.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. Gas chromatograph equipped with an electron capture
detector.
3.1.2. A number of GC columns are available and adequate to
separate the EDB from the xylene solvent. The column used in this
study was a
3.1.3. An electronic integrator or other suitable method of measuring peak areas.
3.1.4. Two-milliliter vials with
3.1.5. Microliter syringe, 10-µL for preparing standards,
3.1.6. Pipets for diluting standards. A Glenco 1-mL dispenser is used to dispense the solvent for desorption.
3.1.7. Volumetric flasks, for preparing standards.
3.2. Reagents
- 3.2.1. Xylene, chromatographic grade.
3.2.2. Ethylene dibromide, reagent grade.
3.2.3. Argon/methane (95%/5%), purified GC grade carrier gas.
3.3. Sample Preparation
- 3.3.1. The front and back sections of each sample are
transferred to separate
3.3.2. Each section is desorbed with 1.0 mL of xylene.
3.3.3. The vials are sealed immediately and allowed to desorb for 30 min with intermittent shaking.
3.4. Standard preparation
- 3.4.1. Standards are prepared by diluting pure EDB with xylene.
3.4.2. A standard containing 1 L of EDB per 100 mL of xylene
equals 2.2 mg/m3 for a
3.5. Analysis
- 3.5.1. GC conditions
| chromatogram: | Figure 3.5.1. |
| argon/methane flow rate: | 11 mL/min |
| oven temperature: | 125°C |
| injector: | 200°C |
| detector: | 150°C |
| EC detector: | Ni-63 |
3.5.2. Peak areas are measured by an electronic integrator or equivalent.
3.5.3. Since it is an external standard method, duplicate injections for all standards and samples are necessary.
3.6. Interferences
- 3.6.1. Any substance having the same general retention time as
EDB is an interference.
3.6.2. GC parameters may be changed to circumvent most interferences.
3.6.3. Retention time on a single column is not considered proof of chemical identity. Samples should be confirmed by GC/MS or other suitable means when needed.
3.7. Calculations
The integrator is usually programmed to report results in
mg/m3 based on a
| mg/m3 = (A)/(0.1)(B) | where | A = mg/m3 on report B = actual air volume (L) |
3.8. Safety precautions
- 3.8.1. All work done with solvents should be done in a hood.
3.8.2. Gloves should be worn when working with ethylene dibromide. Avoid skin contact with all solvents.
3.8.3. Safety glasses should be worn throughout the procedure.
4. Backup Data
- 4.1. Detection limit of the analytical
procedure:
Standard 2.5 × 10-5 µL/mL × 2.175 mg/µL × 1 µL = 54 pg/injection
|
| |
| injection | area |
|
| |
| 1 | 165800 |
| 2 | 94720 |
| 3 | 117200 |
| 4 | 91000 |
| 5 | 114400 |
| 6 |
132900 |
| SD = 27500 CV = 0.23 |
|
|
| |
4.2. Instrument response to analyte and analytical precision
Coefficient of variation of analytical procedure over the range
equivalent to 0.125 to 0.5 mg/m3 for a
|
|
|||
| mg/m3 | 0.5 | 0.25 | 0.125 |
|
|
|||
| area counts | 7872000 | 4035000 | 2139000 |
| 7584000 | 4105000 | 2069000 | |
| 7780000 | 4671000 | 2068000 | |
| 7686000 | 4039000 | 2032000 | |
| 7730500 | 4212500 | 2077000 | |
| SD | 123713 | 307347 | 44773 |
| CV | 0.016 | 0.073 | 0.022 |
|
| |||
4.3. Sensitivity
Figure 4.3.1. represents the calibration curve. The slope of the curve indicates the sensitivity.
4.4. Desorption efficiency
Samples representing 0.55 mg/m3, 1.1
mg/m3, and 2.2
mg/m3 based on
|
|
|||
| mg/m3 | 0.55 | 1.1 | 2.2 |
|
|
|||
| desorption | 85.8 | 87.7 | 85.6 |
| efficiency, | 83.6 | 86.6 | 83.5 |
| % | 86.1 | 86.9 | 82.0 |
| 86.9 | 89.1 | 84.5 | |
| 88.1 | 89.5 | 86.9 | |
| 84.9 | 89.3 | 83.9 | |
| 85.9 | 88.2 | 84.4 | |
|
| |||
4.5. Samples were generated at a concentration of 1 mg/m3 and 80% relative humidity. A time study was then conducted with the following results. The results of the storage tests are shown graphically in Figures 4.5.1. and 4.5.2.
|
| ||||||||
| storage time | % recovery | |||||||
| (days) | (ambient, 20-24°C) | (refrigerated, -5°C) | ||||||
|
| ||||||||
| 0 | 93.7 | 83.8 | 83.2 | 93.7 | 83.8 | 83.2 | ||
| 0 | 82.6 | 84.3 | 80.7 | 82.6 | 84.3 | 80.7 | ||
| 3 | 79.6 | 75.3 | 68.8 | 70.9 | 82.3 | 77.3 | ||
| 7 | 62.9 | 59.7 | 64.6 | 81.3 | 79.4 | 76.3 | ||
| 10 | 76.5 | 71.3 | 73.4 | 86.0 | 81.3 | 83.4 | ||
| 13 | 86.5 | 79.3 | 71.6 | 81.5 | 86.2 | 77.2 | ||
| 16 | 73.6 | 73.7 | 70.0 | 70.6 | 77.1 | ---- | ||
|
| ||||||||
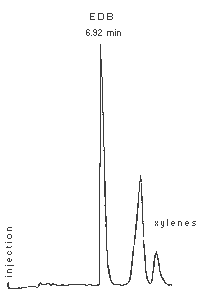
Figure 3.5.1. Chromatogram of a standard of EDB.
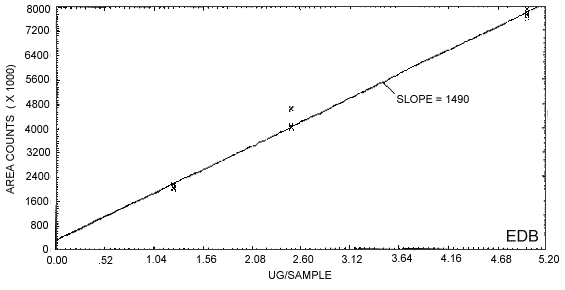
Figure 4.3.1.
Calibration curve of instrument response to EDB.
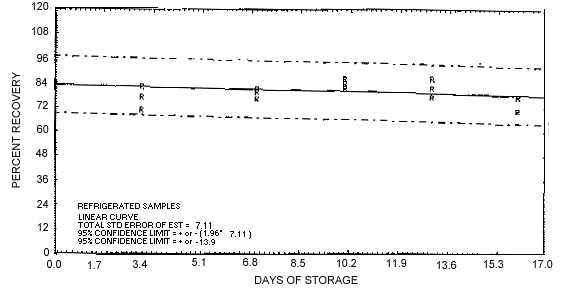
Figure 4.5.1. Refrigerated storage test of EDB.
5. References
5.2. General Industry Safety and Health
Standards. OSHA 2206 (29 CFR 1910) Revised 1/76.
5.3. "Current Intelligence Bulletin 3" NIOSH
7/75.
5.4. "Current Intelligence Bulletin 23" NIOSH
4/78.
5.5. The Condensed Chemical Dictionary 8th
Edition, 1971.
Analysis of
Low Levels of Ethylene Dibromide
Collected on Coconut Shell Charcoal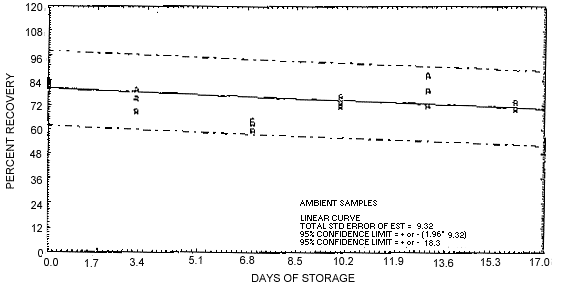
Figure 4.5.2. Ambient storage test for EDB.
5.1. "Criteria for a Recommended
Standard....Occupational Exposure to Ethylene Dibromide" NIOSH 8/77.
| November 1981 | Chemist: Carl J. Elskamp |
Organic Methods Evaluation Branch
OSHA Analytical
Laboratory
Salt Lake City, Utah
1. Discussion
A method for collecting ethylene dibromide (EDB) on activated charcoal
and analyzing the desorbed samples by gas chromatography with an electron
capture detector has previously been evaluated and written up as OSHA's
Organic Methods Evaluation Branch Method Number 2. The target level
concentration for this method was 0.13 ppm (1
mg/m3) for a
In Method Number 2, xylene was used as the desorption solvent. When
xylene was tried at the lower levels, the recovery was low and
inconsistent throughout the range of 0.5 to 125 ppb for a
Various other solvents and solvent mixtures were tested to enhance recovery. These included hexane and benzene and mixtures of carbon disulfide (CS2) in hexane and benzene. It was found that a solution containing 10% (by vol.) CS2 in benzene gave consistently high recoveries. Using this solvent system, the following limit defining parameters were obtained:
2. Limit Defining Parameters
- 2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 38 pg per injection. This amount of EDB gives a peak larger than five times the baseline noise, but due to interferences from the desorption solvent, it was taken to be the detection limit. (Section 3.1.)
2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 41 ng per sample (0.54 ppb/4.1 µg/m3). This is the amount of EDB that can be spiked onto the sampling device which allows for recovery of the amount of EDB equivalent to the detection limit of the analytical procedure. (Section 3.2.)
2.3. Reliable quantitation limit
The reliable quantitation limit is the same as the detection limit of the overall procedure since the recovery is higher than 75% and within ±25% at the 95% confidence interval. (Section 3.3.)
2.4. Recovery
The average recovery of EDB from spiked samples equivalent to 0.5
ppb for a
3. Backup Data
- 3.1. The detection limit of the analytical procedure was
determined by making a
| analytical column: | 1/8 in. × 10 ft, stainless steel, 20% SP-2100/0.1% Carbowax 1500 on 80/100 mesh Supelcoport |
| zone temperatures: | 125°C (column) 200°C (injector) 300°C (detector) |
| carrier gas: | 21 mL/min (nitrogen, for GC/ECD) |
A chromatogram of the detection limit standard and blank solvent are shown in Figure 3.1.
3.2. The analytical detection limit corresponds to 0.5 ppb for a
3.3. The recovery was determined by liquid injection of a standard solution onto charcoal and analyzing the samples the next day.
|
| ||
| sample | % recovery | |
|
| ||
| 1 | 94.2 | |
| 2 | 92.7 | |
| 3 | 92.0 | |
| 4 | 90.7 | |
| 5 | 90.0 | |
| | ||
| SD = 1.67 | ||
|
| ||
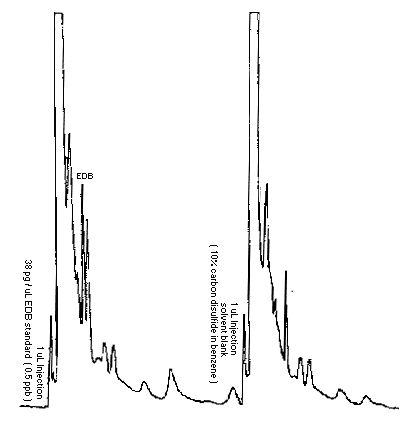
Figure 3.1. Chromatograms at the analytical detection
limit of EDB and of solvent blank.