CHLOROMETHYL METHYL ETHER (CMME)
BIS-CHLOROMETHYL ETHER (BCME)
| Method no.: | 10 |
| Matrix: | Air |
| Target concentration: | 1.0 ppb (4 µg/m3 BCME, 3.2 µg/m3 CMME) |
| Procedure: | Collection in two "derivatizing reagent" bubblers connected in series. The derivative is extracted with hexane and analyzed by gas chromatography using an electron capture detector. |
| Detection limit based | |
| on recommended air volume: | BCME 1 µg/m3, CMME 0.8 µg/m3 (For analytical method only) |
| Recommended air volume and sampling rate: |
50 L at 0.5 L/min |
| Coefficient of variation: | BCME 0.057, CMME 0.068 For analytical methods only, over the range 2 µg/m3 to 8 µg/m3. (50 L air volume) |
| Special requirements: | VERY POTENT CARCINOGENS. Handle with extreme care. Reagents must be pure or serious interferences may occur. |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: August 1979 | Chemist: Dee Chambers |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
The carcinogenicity of bis-chloromethyl ether (BCME) and chloromethyl methyl ether (CMME) (Section 1.1.2.), and the potential presence in the workplace of these compounds has become a major concern for those industries which use or produce these compounds, and for OSHA, the agency charged with ensuring workers a safe and healthful workplace. To determine their actual existence and concentration in suspected industries an air monitoring procedure is essential. Many monitoring techniques have been described in the literature. Some of these techniques are:
- A) Solid adsorbent - GC. The components are allowed to adsorb
on a solid packing and then either thermally desorbed and
reabsorbed at the head of a GC column, or desorbed from the solid
packing with a solvent and analyzed through a GC column. (Refs. 5.1.
- 5.5.)
B) On Column Concentration - GC. The components are allowed to adsorb on the front section of a GC analytical column at a low temperature after which the column temperature is raised and the components eluted and detected. (Ref. 5.6.)
C) Solid adsorbent - GC/MS or MS. The components are allowed to adsorb on a solid packing and then analyzed by GC/MS or by mass spectroscopy alone. (Refs. 5.7. - 5.10.)
D) Solution Derivatization - GC. The components are collected in solutions and reacted to form stable derivatives. The resultant derivatives are analyzed by GC. (Refs. 5.11. and 5.12.)
The preceding methods differ in sensitivity, selectivity, and requirements for training personnel in their operation.
NIOSH has adopted the method described by Solomon and Kallos (Ref. 5.12.), in which a known volume of air is drawn through glass impingers containing a methanolic solution of the sodium salt of 2,4,6-trichlorophenol. CMME and BCME react to form stable derivatives. The derivatives are extracted with hexane and the extract analyzed by GC using an electron capture detector. (Ref. 5.13.) This method has also been recommended by subcommittee 5 of the APHA Intersociety Committee. (Ref. 5.14.)
This method has been used at the OSHA laboratory. However, problems with interferences as well as questions involving the formation of more than one derivative (Ref. 5.15.) have caused concern and were the primary reasons for this evaluation.
In the past, OSHA chemists have found large peaks often masking the BCME and CMME derivatives. It appears that these were the result of reagent impurities or improper extraction technique. It was possible to avoid these interferences by assuring the quality of reagents and by exercising care in the extraction process. The converse was also true, i.e. by using reagents of questionable purity and not removing the extract promptly and carefully, large interfering peaks occurred.
The reasoning for this derivatization method has been considered confusing and chemically unsound, (Ref. 5.15.) primarily due to the formation of more than one product in the derivatization process. This argument is not in itself reason enough to discard a procedure. For a reliable analytical procedure, the reaction products do not need to be stoichiometric as long as the percent conversion to a particular product is constant and reproducible, relative to the amount being determined. This argument is further refuted by the contention that although used by many in the analytical chemistry field, no data have been presented to refute the method. (Ref. 5.16.)
After evaluating portions of alternate procedures, it was decided to evaluate in-depth the recommended NIOSH method.
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
BCME is considered a very powerful carcinogen and is regulated by OSHA in any solid or liquid mixture containing more than 0.1% by weight or volume. Investigations with mice and rats have demonstrated that 0.1 ppm or 1 ppm of BCME in air induced lung cancer (Ref. 5.17.). Investigations at a single factory in which BCME and CMME are widely used revealed a high incidence of lung cancer in CMME workers. The "oat cell carcinoma" found in 12 of 13 examined workers is a relatively rare form of cancer. With one exception the time of exposure was from 3 to 14 years. The average age was 45 years which is far below the general cancer average age of 60 years for male lung cancer. (Ref. 5.18.)
There are no warning properties of BCME exposure; worker exposure by all routes should be carefully controlled.
It is advisable that the laboratory chemist exercise great caution in the use of CMME and BCME. BCME is among those carcinogens for which zero tolerance has been proposed.
CMME is a weaker carcinogen than BCME. However, commercial grade CMME contains 1 to 7% BCME and therefore it should be handled with equal care. (Ref. 5.18.) In addition, it has been found that BCME can form spontaneously whenever formaldehyde and hydrogen chloride coexist in ordinary humid air. (Refs. 5.19. and 5.20.) Furthermore, when CMME is hydrolyzed, formaldehyde and hydrogen chloride are produced. (Ref. 5.22.)
1.1.3. Workplace exposure
Today the use of chloromethyl ethers has been widely curtailed or stopped altogether. They have been used as intermediates in organic synthesis and in the production of anion exchange resins, membranes and other aromatic products. (Ref. 5.7.)
The revelation that BCME could be formed spontaneously upon reaction of formaldehyde and hydrogen chloride indicates a massive industry where exposure could occur.
1.1.4. Physical properties (Refs. 5.21. and 5.22.)
| bis-chloromethyl ether | |
| molecular formula: | ClCH2OCH2Cl |
| molecular weight: | 115 |
| boiling point: | 105°C |
| specific gravity: | 1.315 (20°C) |
| color: | colorless volatile liquid |
| stability: | decomposed by heat and water solubly |
| solubility: | soluble in acetone, benzene, ethyl and methyl alcohol |
| chloromethyl methyl ether | |
| molecular formula: | ClCH2OCH3 |
| molecular weight: | 80.5 |
| melting point: | -103.5°C |
| boiling point: | 59.5°C |
| specific gravity: | 1.0625 (10/4°C) |
| color: | colorless liquid |
| stability: | decomposes in water, soluble in alcohol and ether |
1.2. Detection limit, precision, sensitivity and working range
- 1.2.1. The detection limit for the BCME analytical procedure is
0.05 ng per injection with a coefficient of variation of 0.073 at
this level. The detection limit was determined using 5-µL
injections.
The detection limit for the CMME analytical procedure is 0.04 ng per injection with a coefficient of variation of 0.095 at this level. The detection limit was determined using 5-µL injections.
NIOSH reports a sensitivity of 0.5 ppb for the analytes when a 10-L air sample is used. (Ref. 5.13.)
1.2.2. The pooled coefficient of variation for the analytical method for BCME over a concentration range representing 0.5 to 2 ppb based on the recommended air volume of 50 L was 0.057. The range represented a concentration of 50 to 250 ng/mL. (Section 4.1.)
The pooled coefficient of variation for the analytical method for CMME over a concentration range representing 0.5 to 2 ppb based on the recommended air volume of 50 L was 0.068. The range represented a concentration of 40 to 200 ng/mL. (Section 4.1.)
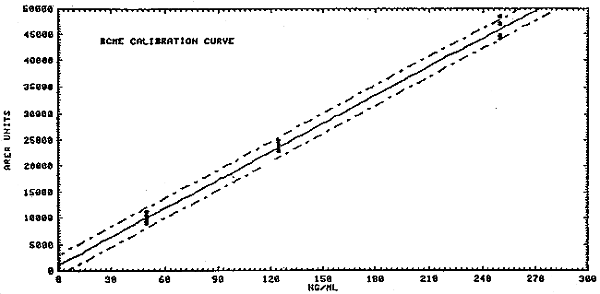
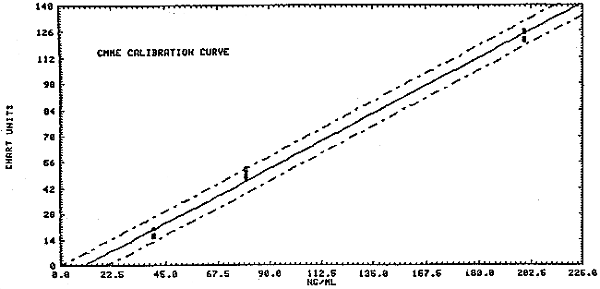
1.2.3. The sensitivity of the analytical procedure over a concentration range representing 0.5 to 2 times the target concentration based on the recommended air volume is 180 area units per ng/mL of BCME and 0.66 chart units per ng/mL of CMME. The sensitivity is the slope of the calibration curve and varies with the particular instrument and type of area measurement used. (Section 4.2.)
1.2.4. The lower limit of the estimated working range is 0.5 ppb in air. This is based on the recommended air volume of 50 L. The upper limit of the working range is dependent on the capacity of the collecting solution.
1.3. Accuracy
- 1.3.1. The overall procedure must provide results that are
within 25% or better at the 95% confidence interval.
1.3.2. The recovery of analyte from the collection medium after storage must be 75% or greater.
1.3.3. The overall procedure has met the above criteria within the limits of the working capabilities of the laboratory. The laboratory is not equipped to generate BCME and CMME samples and therefore the storage recovery and extraction tests were conducted using spiked samples. (Section 4.3.)
1.4. Advantages
- 1.4.1. The major advantages of this method are the low detection
limits and the simultaneous analysis of both BCME and CMME.
1.4.2. The formation of the derivatives stabilizes BCME and CMME while significantly increasing the sensitivity of the analysis.
1.5. Disadvantages
The method involves liquid sampling, extractions and dilutions.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. Two standard air bubblers, with fritted glass inlets.
2.1.2. Calibrated battery powered pump, capable of drawing an accurate and reproducible volume of air through the impingers at a flow rate of 0.5 L/min is required.
2.1.3. Scintillation vials, 20 mL in size with PTFE-lined caps.
2.2. Reagents
- 2.2.1. An analytical grade of contaminant free water produced by
distillation or other suitable means.
2.2.2. Sodium methoxide, AR grade.
2.2.3. Methanol, distilled in glass.
2.2.4. 2,4,6-Trichlorophenol, (TCP) mp 67-68°C (Pure TCP is in the form of white crystals. It is essential that only pure TCP be used).
2.2.5. Derivatizing reagent: Sixteen grams of TCP and 4.4 g of sodium methoxide dissolved in 1 L of methanol. The derivatizing reagent should be analyzed to confirm it is free of contaminants before used in sampling.
2.3. Cleaning of equipment
All glassware used for the analysis must be thoroughly washed, rinsed with distilled water, and dried. The impinger assemblies can be rinsed with reagent grade methanol for repeated use.
2.4. Collection of samples
- 2.4.1. CMME and BCME in air are sampled at a rate of 0.5 L/min
(up to 2 h, if necessary) through the two bubblers connected in
series, each containing 10 mL of the derivatizing solution.
Recommended air volume is 50 L.
2.4.2. Teflon tubing should be used for the connection of the two bubblers in series. Rubber tubing may be used for the connection of the second bubbler to the intake of the pump.
2.4.3. Refill bubblers with additional derivatizing solution if it becomes necessary due to evaporation of original solution.
2.4.4. Transfer the bubbler solutions to separate vials after sampling.
2.4.5. Seal with OSHA labels and ship to laboratory.
2.5. Retention efficiency
At a sampling rate of 1 L/min, humid air (75-80%) at 26°C was drawn through spiked samples to determine if any carry-over occurred. Following 100 L of air, the solution still contained the same amount of BCME or CMME derivative as it did initially. The collection solution does evaporate with time as air is bubbled through, and with prolonged sampling more solution may need to be added.
2.6. Extraction efficiency
The extraction of the derivative from the collection solution is not affected by drawing humid air through the solution. Because an independent method of generation was not available, it is not known what the extraction efficiency is; however, the extraction appears to be constant.
2.7. Recommended air volume and sampling rate
- 2.7.1. The recommended air volume is 50 L.
2.7.2. The recommended sampling rate is 0.5 L/min.
2.8. Interferences (sampling)
- 2.8.1. Interferences can be expected from highly halogenated
organic compounds or compounds that may produce the same derivative.
2.8.2. The known components used in chloromethylation processes do not interfere with the determination of BCME or CMME.
2.8.3. The quality of the reagents and in particular, the 2,4,6-trichlorophenol is important since impurities may be extracted and interfere with the analysis.
2.9. Safety precautions
- 2.9.1. BCME and CMME are carcinogens. Any sampling done in areas
where these compounds are present should be done with extreme
caution, observing safeguards and safety precautions necessary in
these areas.
2.9.2. Although the derivatives formed in the collecting solution are probably less of a health threat than the precursors, any equipment used in the sampling process should be handled as if contaminated by carcinogens.
2.9.3. The toxic effects of the derivatives are not known and therefore should be handled as if they too are carcinogens.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. Hot water bath. Any bath capable of maintaining a
temperature of 65-90°C is adequate.
3.1.2. Gas chromatograph, equipped with a Ni-63 electron capture detector.
3.1.3. Gas chromatograph column. A 6-ft (1.83-m) × 1/4-in. (6.35-mm) glass column packed with 100/120 mesh textured glass beads (GLC-100) which are coated with a two component stationary phase consisting of 0.1% by weight QF-1 and 0.1% by weight OV-17. The column is designed for on-column injection. The packed column is preconditioned at 160°C overnight with 5% methane/ 95% argon at a flow rate of 30-50 1 mL/min.
3.1.4. Strip chart recorder, 1.0 millivolts full scale range.
3.1.5. Hamilton microsyringes.
3.1.6. Assorted laboratory glassware, pipettes, graduated cylinders, etc.
3.1.7. Laboratory shaker.
3.2. Reagents
- 3.2.1. Methanol and hexane, distilled in glass.
3.2.2. Chloromethyl methyl ether, bp 55-58°C.
3.2.3. bis-Chloromethyl ether, bp 100-102°C.
3.2.4. Sodium hydroxide.
3.3. Standard preparation
- 3.3.1. Two microliters of CMME and BCME are added to 50 mL of
hexane. The weights of the components are obtained by using specific
gravities of 1.06 g/mL for CMME and 1.315 g/mL for BCME. This
concentrated standard is then used for preparing a standard curve.
Both of these compounds should be handled in a well ventilated hood.
3.3.2. Ten milliliters of the derivatizing reagent (Section 2.2.5.) is pipetted into five 20-mL scintillation vials. Ten, five, two, one and zero microliters of the concentrated standard are added. These volumes are equivalent to 0.50, 0.25, 0.10, and 0.05 µg of BCME and 0.40, 0.20, 0.08 and 0.04 µg of CMME respectively.
3.3.3. The vials are capped loosely and placed on a steam bath for 5 min. The standard is cooled and 10 mL of 2.0 N NaOH and 2 mL of hexane are pipetted into the vials. Then the standards are shaken for 15 min.
3.4. Sample preparation
- 3.4.1. The sampling solutions are transferred to vials that are
capped loosely and placed in a hot water bath for 5 min (any bath
capable of maintaining a temperature of 65-90°C is suitable).
3.4.2. The samples are allowed to cool. Ten milliliters of 2.0 N NaOH and 2.0 mL of hexane are pipetted into the vial.
3.4.3. The samples are shaken for 15 min and then allowed to stand for a few minutes to allow the phases to separate.
3.4.4. Using extreme care, transfer a portion of the hexane phase to a 2-mL vial. Cap the vial with a PTFE-lined septum cap.
3.5. Analysis
- 3.5.1. GC conditions
The following are the recommended starting instrumental conditions. A gas chromatograph with a Ni-63 electron capture detector is equipped with a 6-ft (1.83-m) × 1/4-in. (6.35-mm) glass column packed with 100-120 mesh textured glass beads (GLC-100) coated with a two component stationary phase consisting of 0.1% by weight QF-1 and 0.1% by weight OV-17. The column is installed for on-column injection. The flow rate of the purified 5% methane/95% argon is 30 mL/min. The temperature of the sample injection zone is adjusted at 175°C and that of the detector at 250°C. The column oven is operated isothermally at 149°C. An oxygen filter is required on the carrier gas.
3.5.2. Injection
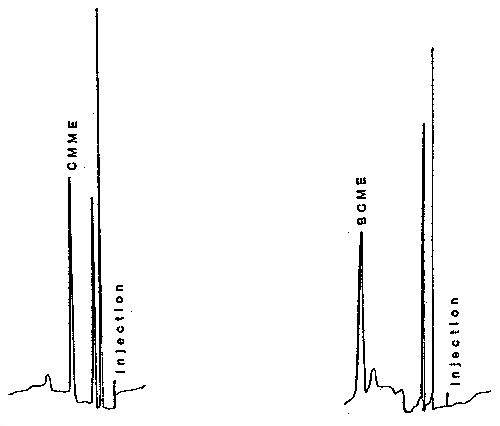
A 2-µL aliquot of the hexane extract is injected into the GC. A complete chromatogram should be obtained in about 10 min. Chromatograms for CMME and BCME are shown in Figure 3.5.2. Replicate injections of each sample and a standard should be made.
3.6. Calculations
- 3.6.1. Standard curves are established by plotting the peak
height or area response versus concentration in nanograms.
3.6.2. Determine the concentration of the desired component in nanograms from the calibration curve.
3.6.3. The concentration of CMME and BCME in the sampled atmosphere can be calculated in ppb at 25°C and 760 mm Hg.
| ppb = | Dx × 24.46
Vs × MW |
| where | D | = | total ng concentration as determined in Section 3.6.2. |
| 24.46 | = | molar volume at 25°C and 760 mm Hg | |
| Vs | = | volume of air sampled in liters | |
| MW | = | molecular weight, CMME - 80.5, BCME - 115 |
3.7. Interferences (analytical)
- 3.7.1. The known components used in chloromethylation processes
do not interfere with the determination of CMME or BCME.
3.7.2. Interferences can be expected from highly halogenated organic compounds or any compound that produces the same derivatives as the analytes.
3.7.3. The purity of 2,4,trichlorophenol is important since impurities can be extracted with hexane and seriously interfere with the chromatographic analysis.
3.8. Safety precautions
- 3.8.1. BCME and CMME are carcinogens. Any work done with the
pure standards should be done in a high efficiency hood or glove box
using all necessary and required safety precautions.
3.8.2. Although the derivatives may be less toxic, they too should be handled with extreme care.
3.8.3. Handle all samples and equipment that comes from the field as if it were contaminated with BCME or CMME.
4. Backup Data
- 4.1. Precision of analytical procedure
The pooled coefficient of variation for the analytical method, for each analyte, was determined from multiple injections of analytical standards. These standards were equivalent to 0.5, 1, and 2 times the target concentration based on the recommended sampling and analytical conditions.
Precision
of the Analytical Method for BCME
|
| |||
| ng/mL | 50 | 125 | 250 |
|
| |||
| area | 11335 | 22719 | 44626 |
| counts | 10068 | 24759 | 44875 |
| 10486 | 23199 | 44380 | |
| 9164 | 23295 | 48533 | |
| 9118 | 23985 | 47154 | |
| 10278 | |||
| 9592 | |||
| 9919 | |||
| 9995 | 23591 | 45914 | |
| SD | 732 | 794 | 1836 |
| CV | 0.073 | 0.034 | 0.040 |
|
| |||
Precision of the
Analytical Procedure for CMME
|
| |||
| ng/mL | 40 | 80 | 200 |
|
| |||
| chart | 17 | 47 | 125 |
| units | 16 | 49 | 121 |
| 20 | 53 | 122 | |
| 16 | 51 | 126 | |
| 17 | |||
| 17.2 | 50 | 123.5 | |
| SD | 1.64 | 2.58 | 2.38 |
| CV | 0.096 | 0.052 | 0.019 |
|
| |||
4.2. Sensitivity
BCME
| = | = | |||
| see Figure 4.2.1. | ||||
CMME
| = | = | |||
| see Figure 4.2.2. | ||||
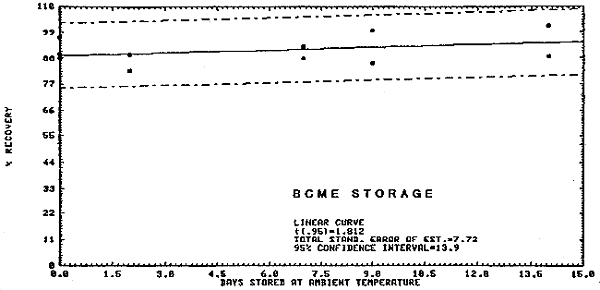
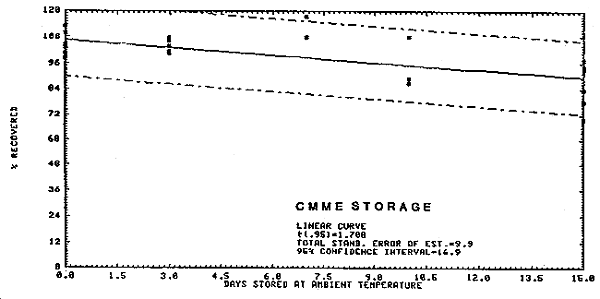
4.3. Storage Tests
The recovery and stability data are represented in Figure 4.3.1. and 4.3.2. for BCME and CMME respectively. Fifty liters of air at 75-80% relative humidity and at room temperature were drawn through each spiked sample. The samples were then stored at room temperature and a portion analyzed every few days. Fresh spiked samples which had no air drawn through them were used as analytical standards. The data are presented below.
BCME Storage Test
|
| |||
| storage time | % recovery | ||
| (days) | |||
|
| |||
| 0 | 88 | 97 | 90 |
| 2 | 90 | 83 | |
| 7 | 98 | 88 | |
| 9 | 86 | 100 | |
| 14 | 89 | 102 | |
|
| |||
CMME Storage Test
|
| ||||||
| Storage time | % recovery | |||||
| (days) | ||||||
|
| ||||||
| 0 | 104.8 | 104.8 | 98.4 | 100.8 | 110.4 | 113.6 |
| 0 | 100.8 | 100.8 | 103.2 | |||
| 3 | 101.6 | 103.9 | 100.8 | 106.2 | 107.8 | |
| 7 | 108.0 | 108.0 | 118.0 | |||
| 10 | 88.9 | 87.1 | 108.4 | |||
| 15 | 78.4 | 84.0 | 70.0 | 92.9 | 94.5 | 97.7 |
|
| ||||||
5. References
- 5.1. D.G. Parkes, et al., Am. Ind. Hyg.
Assoc. J., 37, 165-173(1976).
5.2. L.S. Frankel, R.F. Black, Anal. Chem., 48, 732(1976).
5.3. E.d. Pellizzari, et al., Anal. Letters, 9(1), 4563(1976).
5.4. F. Bruner, et al., Anal. Chem., 50, 53(1978).
5.5. L.G.J.v.d. Ven, A. Venema, Anal. Chem., 51, 1016(1979).
5.6. F.W. Williams, M.E. Limstead, Anal. Chem., 40,2232(1968).
5.7. L. Collier, Environ. Sci. Technol. 6, 930(1972).
5.8. L.A. Shadoff et al, Anal. Chem. 45, 2341(1973).
5.9. K.P. Evans et al, Anal. Chem. 47, 821(1975).
5.10. BCME Analytical Method, H.L.S. 12(4), 403(1975).
5.11. Y. Baba, T. Tanaka, Bull. Chem. Soc. Japan, 51(1), 317(1978).
5.12. R.A. Solomon, G. J. Kallos, Anal. Chem. 47, 955(1975).
5.13. NIOSH Analytical Method P & CAM 220.
5.14. Analytical Method (CMME, BCHE), H.L.S. 13(1)(1976).
5.15. C. Y. Yao, Anal. Chem. 51, 299(1979).
5.16. G. J. Kallos et al., Anal. Chem. 51, 301(1979).
5.17. Federal Register, 39(20) 3557(1974).
5.18. C. Searle, Chemical Carcinogens AMERICAN CHEMICAL SOCIETY, Washington, D.C. 332(1976).
5.19. L.S. Frankel, et al, Environ. Sci. Technol. 8, 356(1974).
5.20. G.J. Kallos, R.A. Solomon, Am. Ind. Hyg. Assoc. J. 469(Nov. 1973).
5.21. G.G. Hawley, The condensed Chemical Dictionary, 8th Ed. (1971).
5.22. N.H. Proctor, J.P. Hughes, Chemical Hazards of the Workplace, J.P. LIPPINCOTT 124, 169 (1978).