CROTONALDEHYDE
| Method no.: | 81 |
| Matrix: | Air |
| Target concentration: | 2 ppm (6 mg/m3) |
| Procedure: | A sample is collected by drawing air through an open face air
monitoring cassette containing two glass fiber filters, each of
which is coated with |
| Recommended air volume and sampling rate: |
6 L at 0.1 L/min |
| Reliable quantitation limit: | 32 ppb (93 µg/m3) |
| Standard error of estimate at the target concentration: (Section 4.7.) |
7.6% |
| Special requirements: | Store samples at -20°C upon receipt at the laboratory. If such storage is not possible, samples must be analyzed within 9 days after collection. (Section 1.2.5.) Keep the samples in the dark whenever possible as a precaution against photodecomposition. |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: April 1990 | Chemist: Warren Hendricks |
Organic Methods Evaluation Branch
OSHA Analytical
Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
This work was performed because there was no fully evaluated OSHA method for the sampling and analysis of crotonaldehyde.
Experiments performed at the OSHA Analytical Laboratory showed
that crotonaldehyde could be collected directly on Carbosieve
An effort was made to extend the sampling method used by OSHA for
the collection of acrolein and formaldehyde (Ref. 5.1.) to include
crotonaldehyde. The method is based on the reaction of
The sampling device used by OSHA to monitor glutaraldehyde (Ref.
5.2.) was tested to determine if it would also efficiently collect
and derivatize crotonaldehyde. That method requires sample
collection using glass fiber filters which have been coated with
| CH3CH=CHCHO + (O2N)2C6H3NHNH2 | ||
| crotonaldehyde | DNPH | |
| (O2N)2C6H3NHN=CHCH=CHCH3
crotonaldehyde-DNPH derivative |
+ H2O
water | |
Initial laboratory experiments showed that the sampling device was effective for the collection and derivatization of crotonaldehyde. The DNPH method was evaluated and it is the basis of this method.
The analysis is performed by HPLC using UV detection. Two
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
Crotonaldehyde can produce toxic effects following ingestion,
inhalation, and adsorption through the eyes or skin. It is an
irritant to the eyes, nose, and throat. It can also cause deep lung
irritation effects which are similar to, but less severe than, those
of phosgene and acrolein. At least one case of sensitization has
been reported. The
Crotonaldehyde has been shown to cause liver tumors in laboratory rats (Ref. 5.5.).
Crotonaldehyde has been identified by the German MAK Commission as a chemical suspected of having carcinogenic potential (Ref. 5.6.).
1.1.3. Workplace exposure
Crotonaldehyde can exist as either the trans or the cis isomer.
Commercial crotonaldehyde is more than 95% trans isomer. The largest
use for crotonaldehyde is in the manufacture of
1.1.4. Physical properties (Refs. 5.3. and 5.4.)
| CAS nos.: | 123-73-9 (trans isomer) 4170-30-3 (inhibited solution, usually contains about 10% water) |
The following physical properties are for CAS no. 123-73-9.
| molecular weight: | 70.1 |
| physical description: | colorless, flammable liquid with a pungent odor, turns pale yellow when exposed to air or light |
| specific gravity: | 0.8531 at 20°C |
| boiling point: | 102°C at 101 kPa (760 mmHg) |
| melting point: | -76.5°C |
| vapor pressure: | 4 kPa (30 mmHg) at 20°C |
| flash point: | 55°F (open cup) |
| explosive limits: | 2.95 and 15.5% by vol. in air |
| chemical formula: | CH3CH=CHCHO |
| synonyms: | b-methylacrolein; propylene
aldehyde; crotonic aldehyde;
|
| The analyte air concentrations listed throughout this method are based on an air volume of 6 L and a solvent extraction volume of 3.0 mL. Air concentrations listed in ppm are referenced to 25°C and 101 kPa (760 mmHg). The analyte concentrations are listed as crotonaldehyde even though the derivative is the actual species analyzed. |
1.2. Limit defining parameters
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 2.85 ng per injection. This is the amount of crotonaldehyde which will give derivative peaks with heights about 5 times the height of the baseline noise. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 0.56 µg per sample (32 ppb or 93 µg/m3). This is the amount of crotonaldehyde spiked on the sampling device which allows recovery of an amount of analyte equivalent to the detection limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 0.56 µg per sample (32 ppb or 93 µg/m3). This is the smallest amount of analyte which can be quantitated within the requirements of a recovery of at least 75% and a precision (±1.96 SD) of ±25% or better. (Section 4.3.)
| The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters. |
1.2.4. Instrument response to the analyte
The instrument response over the concentration range of 0.5 to 2 times the target concentration is linear. (Section 4.4.)
1.2.5. Recovery
The recovery of crotonaldehyde from samples used in an 18-day
storage test remained above 75% for the first 9 days of storage when
the samples were stored at about 22°C. (Section 4.5. and regression
line of Figure 4.5.1.).
The recovery of crotonaldehyde from samples used in an
1.2.6. Precision (analytical procedure)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1, and 2 times the target concentration is 0.014. (Section 4.6.)
1.2.7. Precision (overall procedure)
The precision at the 95% confidence level for the 18-day ambient temperature storage test is ±14.9%. (Section 4.7.) This includes an additional ±5% for pump error.
1.2.8. Reproducibility
Six samples, collected from a controlled test atmosphere, were submitted to the OSHA Analytical Laboratory for analysis. The samples and a draft copy of this procedure were assigned to a chemist who was unassociated with this evaluation. No individual sample deviated from its theoretical value by more than the ±14.9% precision reported in Section 1.2.7. (Section 4.8.)
1.3. Advantage
This sampling and analytical procedure provides a simple and convenient means to monitor occupational exposure to crotonaldehyde.
1.4. Disadvantages
- 1.4.1. Recovery of crotonaldehyde from samples used in an
ambient temperature storage test fell below 75% after 9 days of
storage.
1.4.2. The coated filters are not commercially available.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1 Samples are collected by use of a personal sampling pump
that can be calibrated to within ±5% of the recommended flow rate
with the sampling device in line.
2.1.2. A sample is collected using an open face air monitoring cassette containing 2 glass fiber filters. The filters are separated and retained using cassette center sections (Figure 4.11.). Each filter is coated with DNPH and phosphoric acid. Instructions for the preparation of the coated filters and assembly of the sampler are given in Section 4.11. of this method.
2.2. Reagents
No sampling reagents are required.
2.3. Technique
- 2.3.1. Remove the inlet section (cover) and the end plug on the
exit section of the air monitoring cassette so that sampling is
performed open face.
2.3.2. Attach the sampling device to the sampling pump with flexible, plastic tubing such that the front filter of the sampler is exposed directly to the atmosphere.
2.3.3. Attach the open face air monitoring cassette vertically (face down) in the worker's breathing zone in such a manner that it does not impede work performance or safety.
2.3.4. Remove the sampling device after sampling for the
appropriate time. Replace the inlet section (cover) and the end plug
on the exit section of the air monitoring cassette. Wrap the sample
2.3.5. Ship samples to the laboratory within a day after
collection or store them at
2.3.6. Submit at least one blank with each set of samples. The blank should be handled the same as the other samples except that no air is drawn through it.
2.3.7. List any potential interferences on the sample data sheet.
2.4. Sampler capacity
- 2.4.1. Sampler capacity was evaluated by sampling controlled
test atmospheres with several of the recommended sampling devices
for increasing periods of time. Percent breakthrough was measured as
the amount of crotonaldehyde found on the back filter relative to
the total amount collected on the entire sampling device.
2.4.2. An additional sampler capacity experiment was performed at reduced relative humidity to determine if low humidity had an effect on capacity. Five samples were collected for 1 h at 0.1 L/min from a controlled test atmosphere containing 12 mg/m3 of crotonaldehyde at 36% relative humidity and 26°C. The average amount of crotonaldehyde recovered from the five samples was 97% of theoretical and the average breakthrough was 0.2%.
2.5. Extraction efficiency
- 2.5.1. The extraction efficiency for crotonaldehyde from DNPH
coated glass fiber filters at the target concentration was 96.6%.
(Section 4.10.)
2.5.2. Extracted samples remain stable for at least 16 h. (Section 4.10.)
2.6. Recommended air volume and sampling rate
- 2.6.1. For long-term samples, collect 6 L at 0.1 L/min.
2.6.2. For short-term samples, collect 1.5 L at 0.1 L/min.
2.6.3. When short-term samples are required, the reliable quantitation limit becomes larger. For example, the reliable quantitation limit is 130 ppb (373 µg/m3) for crotonaldehyde when 1.5 L of air is collected.
2.7. Interferences (sampling)
- 2.7.1. Any substance, present in the sampled air, that is
capable of reacting with DNPH and thereby depleting the derivatizing
reagent is a potential interference. Many aldehydes and ketones are
capable of reacting with DNPH.
2.7.2. Suspected interferences should be reported to the laboratory with submitted samples.
2.8. Safety precautions (sampling)
- 2.8.1. Attach the sampling equipment to the worker in such a
manner that it will not interfere with work performance or safety.
2.8.2. Follow all safety practices that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A high-performance liquid chromatograph (HPLC) equipped
with a UV detector and a manual or automatic sample injector. The
following Waters Associates equipment was used in this evaluation: a
Model 6000A HPLC pump, a Model 440 UV detector, and a WISP 710B
automatic sample injector.
3.1.2. An HPLC column capable of resolving the
crotonaldehyde-DNPH derivative from interferences. Either a DuPont
Zorbax CN column
3.1.3. An electronic integrator or some other suitable means to
measure detector response. A
3.1.4. Vials, 4-mL glass with Teflon-lined septum caps.
3.1.5. Volumetric flasks, pipets and syringes for preparing standards, making dilutions and performing injections.
3.1.6. Pipets, disposable, Pasteur-type.
3.1.7. A tube rotator or other suitable means to agitate the
samples during extraction. A Fisher
3.2. Reagents
- 3.2.1. Acetonitrile, HPLC grade. American Burdick and Jackson
acetonitrile UV was used in this evaluation.
3.2.2. Water, HPLC grade. Water from a Millipore Milli-Q water filtration system was used in this evaluation.
3.2.3. Phosphoric acid, reagent grade. "Baker Analyzed" Reagent grade 85% phosphoric acid was used in this evaluation.
3.2.4. Crotonaldehyde. Aldrich Chemical Company, predominately trans, 99+% Gold Label grade crotonaldehyde, lot no. 06226CT, was used in this evaluation.
3.2.5. 2,4-Dinitrophenylhydrazine (DNPH). DNPH (70%), lot no. 1707LJ, was obtained from Aldrich Chemical Company and was recrystallized from hot acetonitrile for use in this evaluation.
3.2.6. Analytical standard preparation solution. This solution is prepared by diluting 0.33 g of recrystallized DNPH and 0.9 mL of phosphoric acid to 250 mL with acetonitrile.
3.3. Standard preparation
- 3.3.1. Prepare analytical standards about 24 h before the air
samples are to be analyzed so that the ratio of the
3.3.2. Prepare crotonaldehyde stock standard solutions by diluting 99% crotonaldehyde with acetonitrile. A standard containing 3.35 mg/mL of crotonaldehyde was prepared by diluting 169.2 mg of the 99% material to 50 mL with acetonitrile.
3.3.3. Place 3.0-mL aliquots of analytical standard preparation
solution (Section 3.2.6.) into each of several
3.3.4. Prepare analytical standards by injecting appropriate
volumes of crotonaldehyde stock standard solutions (Section 3.3.2.)
into the sealed
3.3.5. Prepare a sufficient number of standards to generate a calibration curve. Analytical standard concentrations must bracket sample concentrations.
3.4. Sample preparation
- 3.4.1. Open the air monitoring cassette and remove the front
coated filter. Transfer the filter to a
3.4.2. Add 3.0 mL of acetonitrile to each vial.
3.4.3. Seal the vials with Teflon-lined septum caps and place
them on the tube rotator. Rotate the samples for 1 h at 60 rpm.
Samples do not require the
3.5. Analysis
- 3.5.1. HPLC conditions
| column: | J.T. Baker Bakerbond CN, 25 cm × 4.6-mm i.d. |
| mobile phase: | 40% acetonitrile in water containing 0.1% phosphoric acid (v/v/v) |
| flow rate: | 1 mL/min |
| injection volume: | 15 µL |
| UV detector: | 365 nm |
| retention times: | 6.5 and 9.2 min |
Standards have approximately a 1 to 1 ratio of the two derivative peaks following the required 24 h equilibration time. Air samples may contain predominantly the 9.2 min peak depending on their storage history.
3.5.2. A chromatogram at the target concentration is shown in Figure 3.5.2.
3.5.3. Use a suitable method such as electronic integration to measure detector response (peak areas or heights).
3.5.4. Program the integrator to add the detector responses of
the two
3.5.5. Prepare a calibration curve by plotting the summed
integrator result for each standard solution against its respective
actual concentration (in micrograms per standard). Determine the
3.6. Interferences (analytical)
- 3.6.1. Any compoud having a similar retention time as the
3.6.2. HPLC parameters (mobile phase composition, column, analytical wavelength, etc.) may be changed to circumvent interferences.
3.6.3. Retention time on a single column is not proof of chemical identity. Analysis using an alternate HPLC column, detection at another wavelength, comparison of absorbance response ratios, and structure determination by mass spectrometry are additional means of identification.
3.7. Calculations
- 3.7.1. The concentration (micrograms of crotonaldehyde per
sample) of samples is determined from the calibration curve. If
crotonaldehyde is found on the back filter, it is added to the
amount found on the front filter. Blank corrections should be
performed before adding the results together.
3.7.2. The crotonaldehyde air concentration can be expressed using the following equation:
mg/m3 = A / (B)(E)
| where | A = | µg/sample from Section 3.7.1. |
| B = | liters of air sampled | |
| E = | extraction efficiency (decimal form) |
3.7.3. The following equation can be used to convert crotonaldehyde results in mg/m3 to ppm at 25°C and 101 kPa (760 mmHg):
ppm = (mg/m3)(24.46) / 70.l
| where | mg/m3 = | result from Section 3.7.2. |
| 24.46 = | molar volume at 101 kPa (760 mmHg) and 25°C | |
| 70.1 = | molecular weight of crotonaldehyde |
3.8. Safety precautions (analytical)
- 3.8.1. Avoid skin contact and inhalation of all chemicals.
3.8.2. Restrict the use of all chemicals to a fume hood.
3.8.3. Wear safety glasses and a lab coat in all lab areas.
4. Backup Data
- 4.1. Detection limit of the analytical procedure
The injection size recommended in the analytical procedure (15 µL) was used to determine the detection limit of the analytical procedure. The detection limit of the analytical procedure was 2.85 ng per injection. This was the amount of crotonaldehyde that gave derivative peaks with heights about 5 times the height of the baseline noise. This detection limit was determined by the analysis of a standard containing 0.187 µg/mL crotonaldehyde. Figure 4.1. is a chromatogram of the detection limit of the analytical procedure.
4.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 0.56 µg per sample (32 ppb or 93 µg/m3). The injection size recommended in the analytical procedure (15 µL) was used in the determination of the detection limit of the overall procedure. Six vials, each containing a coated glass fiber filter, were each liquid spiked with 0.56 µg of crotonaldehyde. The samples were extracted about 16 h after being spiked.
|
| ||
| sample number |
theoretical amount (µg) |
amount recovered (µg) |
|
| ||
| 1 | 0.56 | 0.50 |
| 2 | 0.56 | 0.51 |
| 3 | 0.56 | 0.50 |
| 4 | 0.56 | 0.54 |
| 5 | 0.56 | 0.60 |
| 6 | 0.56 | 0.54 |
|
| ||
4.3. Reliable quantitation limit data
The reliable quantitation limit is also 0.56 µg per sample (32 ppb or 93 µg/m3). The injection size recommended in the analytical procedure (15 µL) was used in the determination of the reliable quantitation limit. Because the recovery of crotonaldehyde from spiked samples (Section 4.2.) was greater than 75% and also because the precision (±1.96 SD) was less than ±25%, the detection limit of the overall procedure and reliable quantitation limit are the same.
|
| ||
| percent recovered |
statistics | |
|
| ||
| 89.3 | ||
| 91.1 | 94.9 | |
| 89.3 | SD = | 6.79 |
| 96.4 | Precision = | (±1.96)(6.79) |
| 107.1 | = | ± 13.3% |
| 96.4 | ||
|
| ||
4.4. Instrument response to crotonaldehyde
The instrument response to crotonaldehyde over the range of 0.5 to 2 times the target concentration is linear with a slope of 117361 area counts per microgram per milliliter. The response to crotonaldehyde was determined by multiple injections of standards. The data in Table 4.4. is presented graphically in Figure 4.4.
|
| |||
| × target concn µg/sample |
0.5× 16.75 |
1× 33.5 |
2× 67.0 |
|
| |||
| area counts | 1888830 | 3794360 | 7765360 |
| 1844160 | 3853520 | 7792780 | |
| 1839390 | 3826430 | 7794280 | |
| 1923890 | 3868140 | 7696830 | |
| 1886350 | 3868160 | 7832800 | |
| 1943860 | 3794140 | 7805420 | |
| 1887747 | 3834125 | 7781245 | |
|
| |||
4.5. Storage test
Eighteen samples were collected on each of two consecutive days by
sampling test atmospheres containing an average of 6.5
mg/m3 crotonaldehyde for 1 h at 0.1 L/min.
The average relative humidity of the atmospheres was 80% at 25°C.
Eighteen of the samples were stored in a freezer at
|
| ||||||||
| storage time (days) |
% recovery (ambient) |
% recovery (freezer) | ||||||
|
| ||||||||
| 0 | 88.5 | 93.2 | 93.9 | 103.4 | 98.2 | 90.2 | ||
| 4 | 88.6 | 96.9 | 95.3 | 101.3 | 95.3 | 92.0 | ||
| 7 | 82.8 | 84.8 | 77.1 | 101.0 | 97.9 | 95.2 | ||
| 11 | 66.4 | 73.1 | 58.2 | 100.0 | 98.9 | 96.6 | ||
| 13 | 97.2 | 98.7 | 98.4 | |||||
| 14 | 60.1 | 67.5 | 67.0 | |||||
| 18 | 58.7 | 55.6 | 52.6 | 101.6 | 99.0 | 98.5 | ||
|
| ||||||||
4.6. Precision (analytical method)
The precision of the analytical procedure is defined as the pooled coefficient of variation determined from replicate injections of crotonaldehyde standards at 0.5, 1, and 2 times the target concentration.
|
| |||
| × target concn µg/sample |
0.5× 16.75 |
1× 33.5 |
2× 67.0 |
|
| |||
| SD1 | 41704.5 | 34440.8 | 46740.8 |
| CV | 0.0221 | 0.00898 | 0.00601 |
|
1 standard deviation is in area counts | |||
4.7. Precision (overall procedure)
The precision of the overall procedure is determined from the storage data. The determination of the standard error of estimate (SEE) for a regression line plotted through the graphed storage data allows the inclusion of storage time as one of the factors affecting overall precision. The SEE is similar to the standard deviation except it is a measure of dispersion of data about a regression line instead of about a mean. It is determined with the following equation:
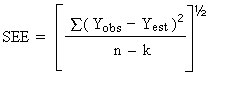
| where | |
| n = k = k = |
total no. of data points 2 for linear regression 3 for quadratic regression |
| Yobs = | observed % recovery at a given time |
| Yest = | estimated % recovery from the regression line at the same given time |
An additional ±5% for pump error is added to the SEE by the
addition of variances. The precision at the 95% confidence level is
obtained by multiplying the SEE (with sampling error included) by 1.96
(the
4.8. Reproducibility
Six samples, collected from a controlled test atmosphere were
assigned to a chemist unassociated with this study. The samples were
stored at ambient temperature for three days before submission to the
laboratory for analysis. The intent of the delay was to simulate
sample shipment from the field to the laboratory. The samples were
analyzed after 47 days of additional storage at about
|
| |||
| µg collected | µg recovered | % recovered | % deviation |
|
| |||
| 34.35 | 34.57 | 100.6 | +0.6 |
| 34.52 | 36.76 | 106.5 | +6.5 |
| 37.79 | 37.36 | 98.9 | -1.1 |
| 32.77 | 32.51 | 99.2 | -0.8 |
| 32.99 | 30.29 | 91.8 | -8.2 |
| 35.42 | 33.80 | 95.4 | -4.6 |
|
| |||
4.9. Sampler capacity
Sampler capacity was evaluated by sampling controlled test
atmospheres with several of the recommended sampling devices for
increasing periods of time. The crotonaldehyde content of the test
atmospheres was 12 mg/m3 and the relative
humidity was 76% at 26°C. Percent breakthrough was measured as the
relative amounts of crotonaldehyde collected on the front and back
filters of the sampling device.
|
| |
| air volume (L) |
breakthrough (%) |
|
| |
| 6.4 | 1.6 |
| 7.4 | 2.4 |
| 8.8 | 6.4 |
| 13.6 | 11.4 |
| 15.1 | 14.8 |
|
| |
4.10. Extraction efficiency and stability of extracted samples
The extraction efficiency for crotonaldehyde was determined by liquid spiking each of six DNPH coated glass fibers contained in separate glass vials with 20 µL of a solution containing 1.675 mg/mL of crotonaldehyde in acetonitrile. These samples were stored at room temperature for 1 h and then extracted and analyzed. The average extraction efficiency was 96.6%. Following the initial analysis, the samples were immediately resealed and reanalyzed about 16 h later using freshly prepared standards. The average of the reanalyzed samples was 97.8% of the original analysis.
|
| |
| extraction efficiency (%) |
reanalysis (%) |
|
| |
| 97.3 | 94.0 |
| 94.6 | 91.5 |
| 96.4 | 94.3 |
| 98.6 | 95.0 |
| 96.8 | 96.4 |
| 96.0 | 96.0 |
|
| |
4.11. Procedure to coat glass fiber filters with DNPH/phosphoric acid and assembly of the sampling device
- 4.11.1. Apparatus
- 4.11.1.1. Hotplate
4.11.1.2. Miscellaneous glassware: 250-mL volumetric
flask, 30-,
4.11.1.3. Plastic air monitoring cassettes, for 37-mm diameter
filters. Unassembled
4.11.2. Reagents
- 4.11.2.1. Acetonitrile, HPLC grade. American Burdick and
Jackson acetonitrile UV was used in this evaluation.
4.11.2.2. 2,4-Dinitrophenylhydrazine (DNPH). DNPH (70%), lot No. 1707 LJ, obtained from Aldrich Chemical Company, was recrystallized from hot acetonitrile for use in this evaluation.
4.11.2.3. Glass fiber filters, 37-mm diameter Gelman Sciences Type A glass fiber filters, lot No. 8318, were used in this evaluation.
4.11.2.4. Phosphoric acid, reagent grade. "Baker analyzed" Reagent grade 85% phosphoric acid was used in this evaluation.
4.11.2.5. DNPH/phosphoric acid solution. Prepare this solution by diluting 2 g of recrystallized DNPH and 5 mL of 85% phosphoric acid to 250 mL with acetonitrile.
4.11.3. Procedure
(CAUTION! Evaporation of acetonitrile must be performed in a fume hood.)
Place a glass fiber filter on a 30-mL beaker, or some other
suitable support, so that only the outside edge of the filter is
supported. Pipet 0.5 mL of the DNPH solution (Section 4.11.2.5.)
onto the surface of the filter. Make sure that the filter is
completely saturated with the solution. Allow the acetonitrile to
evaporate. Store prepared filters in a tightly sealed container at
Assemble the sampling device by placing a coated filter in the outlet section of the air monitoring cassette. Next, place a center support section on the first filter. Now, put another coated filter on the center support section and another center support section on top of that filter. Complete the assembly by placing the inlet section on the center support section. Plug the outlet and inlet openings with plastic end plugs. An exploded view of the air sampler is shown in Figure 4.11. Put the air sampler on a table top with the outlet section down. Press on the top of the air sampler with sufficient force to seal the cassette. Use masking tape or shrink bands to further seal the two center and the outlet sections of the cassette. Store the assembled air sampler at reduced temperature (if possible) when there is a delay of more than a day or two before sampling.
4.12. Generation of controlled test atmospheres
The controlled test atmospheres which were used in this evaluation
were generated by pumping a crotonaldehyde/water solution into a
heated glass manifold with a Sage Instruments Model 355 Syringe Pump.
The crotonaldehyde/water solution was volatilized and then diluted
with heated air. The dilution air was metered into the heated glass
manifold using a precision, calibrated rotameter. The air was
humidified, if desired, by passing it through a water bubbler prior to
its entering the heated glass manifold. The water bubbler was
contained in a
The crotonaldehyde concentration of the test atmosphere was adjusted to the desired level by varying the aldehyde concentration of the crotonaldehyde/water solution.
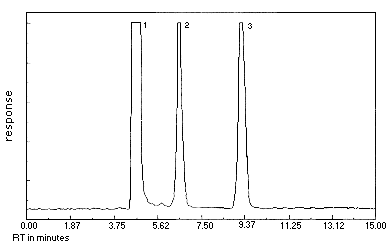
Figure 3.5.2. Crotonaldehyde chromatogram at the target concentration. Peak identification was as follows: 1, DNPH; 2,crotonaldehyde-DNPH (peak 1); 3,crotonaldehyde-DNPH (peak 2).
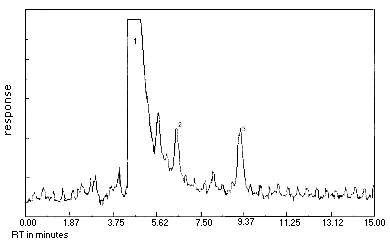
Figure 4.1. Detection limit of the analytical procedure for crotonaldehyde. Peak identification was as follows: 1, DNPH; 2,crotonaldehyde-DNPH (peak 1); 3,crotonaldehyde-DNPH (peak 2).
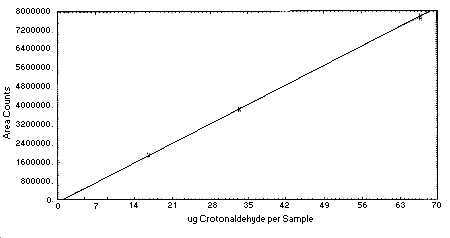
Figure 4.4. Calibration
curve for crotonaldehyde.
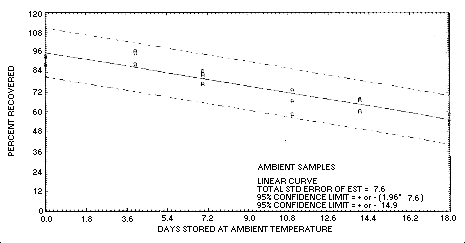
Figure 4.5.1. Ambient
temperature storage test for crotonaldehyde.
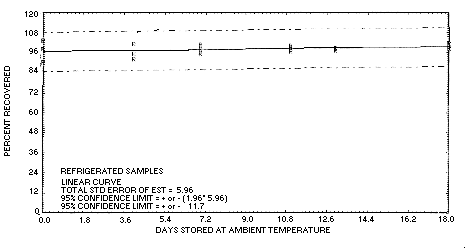
Figure 4.5.2. Refrigerated
temperature storage test for crotonaldehyde.
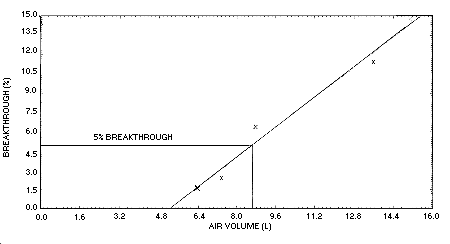
Figure 4.9. Sampler
capacity for crotonaldehyde.
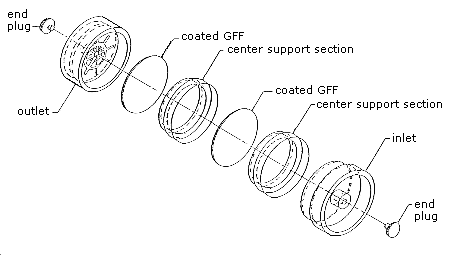
Figure 4.11. Sampling device for crotonaldehyde.
5. References
- 5.1. "OSHA Analytical Methods Manual"; U.S. Department of Labor,
Occupational Safety and Health Administration; OSHA Analytical
Laboratory: Salt Lake City, UT, 1985; Method 52; American Conference
of Governmental Industrial Hygienists (ACGIH): Cincinnati, ISBN:
5.2 Hendricks, W. "OSHA Method No. 64; Glutaraldehyde" OSHA Analytical Laboratory, unpublished, Salt Lake City, UT 84165, June 1987.
5.3. "Documentation of the Threshold Limit Values and Biological
Indices", 5th ed.; American Conference of Governmental Industrial
Hygienists (ACGIH): Cincinnati, ISBN:
5.4. "NIOSH/OSHA Occupational Health Guidelines for Chemical
Hazards", U.S. Dept. of Health and Human Services, Public Health
Services, Center for Disease Control, NIOSH and U.S. Dept. of Labor,
OSHA: U.S. Government Printing Office Washington, DC, Jan 1981,
Crotonaldehyde, DHHS (NIOSH) Publ. No.
5.5. Chung, F.; Tanaka, T.; and Hecht, S. Cancer Res.
1986, 46,
5.6. "Appendix to the Documentation of the Threshold Limit Values
and Biological Indices", 5th ed.; American Conference of Governmental
Industrial Hygienists (ACGIH): Cincinnati, ISBN:
5.7. Baxter, W. In "Kirk-Othmer Encyclopedia of Chemical
Technology", 3rd ed.; Grayson, M., Ed.; John Wiley & Sons, New
York, 1979, Vol. 7, pp