![]()
N-PHENYL-2-NAPHTHYLAMINE (N-PHENYL-b-NAPHTHYLAMINE)
| Method number: | 96 | |
| Matrix: | Air | |
| Procedure: | Samples are collected closed face by drawing known
volumes of air through sampling devices consisting of
| |
| Recommended air volume and sampling rate: |
240 L at 1 L/min | |
|
| ||
| N-Phenyl-1-naphthylamine | N-Phenyl-2-naphthylamine | |
|
| ||
| Target concentration: | 1 ppb (9.0 µg/m3) | 1 ppb (9.0 µg/m3) |
| Reliable quantitation limit: | 17 ppt (150 ng/m3) | 3.0 ppt (27 ng/m3) |
| Standard error of estimate at the target concentration: |
5.2% | 5.3% |
|
| ||
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. | |
| Date: September 1992 | Chemist: Carl J. Elskamp | |
OSHA Salt Lake Technical Center
Salt Lake City, UT 84165-0200
1. General Discussion
- 1.1. Background
- 1.1.1. History
Previous to this evaluation there were no validated air sampling
procedures for
An alternative sampling procedure using
No exposure limits have been established for these two amines by
OSHA or ACGIH, but ACGIH categorizes
The filters are extracted with methyl alcohol and the extract is
analyzed directly by HPLC using a fluorescence detector. The optimum
excitation wavelength is 330 nm for
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
N-Phenyl-1-naphthylamine is a skin, eye, and mucous membrane irritant. Exposure by inhalation may cause respiratory irritation with sore nose, sore throat, and cough. (Ref. 5.10.) The toxicity has not been fully characterized. It is a questionable carcinogen based on experimental data. When heated to decomposition it emits toxic fumes of NOx. (Ref. 5.9.)
N-Phenyl-2-naphthylamine is an eye, skin, and mucous
membrane irritant. Exposure through inhalation may cause sore
throat, shortness of breath, headache, nausea, dizziness, faintness,
unconsciousness, bluish skin and methemoglobinemia. Previously
exposed persons may experience sensitization reactions. When heated
to decomposition it emits toxic fumes of
NOx. (Ref.
5.10.) The International Agency for Research on Cancer has
concluded that there is limited evidence for carcinogenicity of
1.1.3. Workplace exposure
N-Phenyl-1-naphthylamine is used in the production of dyes and other organic chemicals, and also as a rubber antioxidant. (Ref. 5.12.)
N-Phenyl-2-naphthylamine is primarily used as an
antioxidant in rubber processing. It is also used as a stabilizer in
1.1.4. Physical properties and other descriptive information (Ref. 5.10.)
|
| |||
| Property | |||
|
| |||
| CAS number: molecular weight: melting point: boiling point: vapor pressure: specific gravity: |
90-30-2 219.29 55°C 335°C 0.1 mmHg at 20°C 1.23 |
135-88-6 219.29 108°C 395-399°C 15 mmHg at 235°C 1.24 | |
| description: | tan to purple crushed solidor crystals with an
|
grey to tan flakes, rhombic crystals or powder | |
| solubility: | insoluble in water; soluble in acetone, benzene, alcohol | insoluble in water; soluble in benzene, alcohol, ether, actic acid, acetone | |
| synonyms: | |||
| structural formula: | 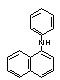 |
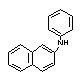 | |
The analyte air concentrations throughout this method are based on the recommended sampling and analytical parameters. Air concentrations listed in ppb and ppt are referenced to 25°C and 101.3 kPa (760 mmHg).
1.2. Limit defining parameters
- 1.2.1. Detection limit of the analytical procedure
The detection limits of the analytical procedure are 180 and 32.5
pg per injection for
1.2.2. Detection limit of the overall procedure
The detection limits of the overall procedure are 36.0 and 6.49
ng per sample for
1.2.3. Reliable quantitation limit
The reliable quantitation limits are 36.0 and 6.49 ng per sample
for
The reliable quantitation limits and detection limits reported in this method are based upon optimization of the instrumentation for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
1.2.4. Instrument response to the analyte
The instrument response over concentration ranges representing 0.5 to 2 times the target concentrations is linear for both analytes. (Section 4.4.)
1.2.5. Recovery
The recoveries of
1.2.6. Precision (analytical method only)
The pooled coefficients of variation obtained from replicate
injections of analytical standards at 0.5, 1, and 2 times the target
concentrations are 0.0043 and 0.0019 for
1.2.7. Precision (overall procedure)
The precisions at the 95% confidence level for the 17-day ambient
storage tests are ±10.2 and ±10.4% for
1.2.8. Reproducibility
Six samples for each analyte, spiked by liquid injection, and a draft copy of this procedure were given to a chemist unassociated with this evaluation. The samples were analyzed after 11 days of storage at approximately 0°C. No individual sample result deviated from its theoretical value by more than the corresponding precision of the overall procedure as reported in Section 1.2.7. (Section 4.8.)
1.3. Advantages
- 1.3.1. The Vitamin C treated glass fiber filter is not only a
very effective air sampling medium for these amines, the collected
amines remain stable, even at room temperatures.
1.3.2. The analysis is rapid, sensitive, and precise.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. Samples are collected using a personal sampling pump that
can be calibrated within ±5% of the recommended flow rate with the
sampling device attached.
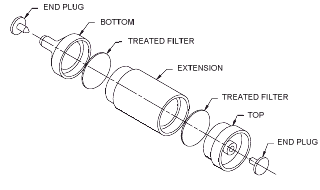 2.1.2.
Samples are collected closed face using a sampling device consisting
of two
2.1.2.
Samples are collected closed face using a sampling device consisting
of two
2.2. Reagents
None required.
2.3. Sampling technique
- 2.3.1. Remove the plastic end plugs from the sampling device
immediately before sampling.
2.3.2. Attach the sampling device to the sampling pump with
flexible,
2.3.3. Immediately after sampling, insert the plastic end plugs into the sampling devices.
2.3.4. Seal and identify each sampling device with an OSHA Form 21.
2.3.5. Submit at least one blank with each sample set. Handle the blanks in the same manner as the air samples, but draw no air through them.
2.3.6. Record the volume of air sampled (in liters) for each sample, along with any potential interferences.
2.4. Collection efficiency
Collection efficiency studies were conducted by drawing humid air
through a sampling device that was attached to a glass
2.5. Extraction efficiency
- 2.5.1. The average extraction efficiencies from six filters for
each amine spiked at the target concentration are 94.9% and 96.7%
for
2.5.2. The stability of extracted samples was verified by
reanalyzing the extraction efficiency samples 24 h later using fresh
standards. The average recoveries for the reanalyzed samples are
92.8% and 92.9% for
2.5.3. Extraction efficiencies must be determined for each lot of treated filters. In order to prevent loss of analyte through photo-oxidation, it is imperative that the filters are immediately protected from light after they are spiked with the amine of interest.
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 240 L.
2.6.2. The recommended sampling rate is 1 L/min.
2.6.3. When short-term samples are required, the reliable
quantitation limits will be larger. For example, the reliable
quantitation limit for
2.7. Interferences (sampling)
- 2.7.1. Any compound in the sampled air that will react with the
Vitamin C on the treated filters or with the collected analyte is a
potential sampling interference.
2.7.2. Suspected interferences should be reported to the laboratory with submitted samples.
2.8. Safety precautions (sampling)
- 2.8.1. Attach the sampling equipment to the employees so it will
not interfere with work performance or safety.
2.8.2. Follow all safety procedures that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. An HPLC equipped with an fluorescence detector. A
3.1.2. An HPLC column capable of separating the amines from the
solvent, Vitamin C and interferences. A Waters
3.1.3. An electronic integrator or some other suitable means of measuring peak areas or heights. A Waters 860 Networking Computer System was used in this evaluation.
3.1.4. Small resealable vials with Teflon-lined caps capable of holding at least 3 mL.
3.1.5. Dispensers or pipets capable of delivering 2.00 mL.
3.1.6. A rotator or rocker to extract the sample filters.
3.2. Reagents
- 3.2.1. N-Phenyl-1-naphthylamine and
3.2.2. L-Ascorbic acid (Vitamin C), reagent grade. The Vitamin C used in this evaluation was from Aldrich Chemical Company, Inc.
3.2.3. Methyl alcohol, HPLC grade. The methyl alcohol used in this evaluation was from Fisher Chemical, Fair Lawn, NJ.
3.2.4. Standard preparation solution, consisting of 5.0 mg/mL of Vitamin C in methyl alcohol. (Note: The dissolution of Vitamin C in methyl alcohol can be hastened by sonication.)
3.2.5. Water, HPLC grade. The water used in this evaluation was
from an
3.3. Standard preparation
- 3.3.1. CAUTION. THESE AROMATIC AMINES SHOULD BE CONSIDERED
CARCINOGENIC. Restrict the use of the pure amines and
concentrated standards to regulated areas. All standards are
prepared and diluted with a preparation solution consisting of 5.0
mg/mL of Vitamin C in methyl alcohol. Prepare concentrated stock
standards by diluting 10 to 50 mg of the pure amines to 10.00 mL.
Prepare intermediate standards by diluting the appropriate volume of
concentrated stock standards to 25.00 or 50.00 mL. Prepare
analytical standards by injecting microliter amounts of intermediate
standards into vials that contain 2.00 mL of the preparation
solution. For example, a 3.380 µg/µL concentrated
stock standard of
3.3.2. Bracket sample concentrations with analytical standard concentrations. If sample concentrations are higher than the upper range of prepared standards, prepare additional standards to ascertain detector response.
3.4. Sample preparation
- 3.4.1. Transfer the sample filters to separate 4-mL vials.
3.4.2. Add 2.00 mL of methyl alcohol to each vial.
3.4.3. Recap and rotate or rock the vials for 10 min.
3.4.4. Analyze the methyl alcohol extract of each sample by HPLC.
3.5. Analysis
- 3.5.1. HPLC conditions and information
| injection volume: | 10 µL |
| column: | Waters Radial-Pak™ 100-mm × 8-mm i.d. Nova-Pak™
C18 cartridge in an
|
| mobile phase: | 85/15, methyl alcohol/water |
| flow rate: | 2 mL/min |
| retention times: | N-Phenyl-1-naphthylamine, 3.15 min
N-Phenyl-2-naphthylamine, 3.21 min (Note: If better separation of the two amines is required, the strength of the mobile phase can be changed. For example, when 68/32, methyl alcohol/water is used, the resulting retention times are 12.0 and 12.8 min for |
| excitation wavelength: | N-Phenyl-1-naphthylamine, 330
nm N-Phenyl-2-naphthylamine, 300 nm |
| emission wavelength: | 370 nm standard long pass filter for both amines |
| response time: | 2 sec |
| photomultiplier voltage: | 1100 V |
| chromatogram at 1× the target concentration: | |
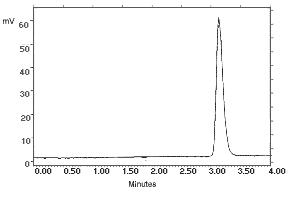 2.172 µg/sample of |
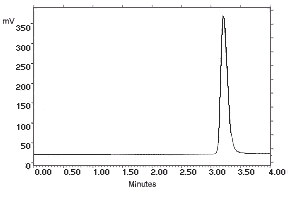 2.163 µg/sample of |
3.5.2. Construct acalibration curve by plotting response (peak areas or heights) of standard injections versus micrograms of analyte per sample. Bracket sample concentrations with standards.
3.6. Interferences (analytical)
- 3.6.1. Any compound that elutes in the same general time as the
amine of interest is a potential interference. Suspected
interferences reported to the laboratory with submitted samples by
the industrial hygienist must be considered before samples are
extracted.
3.6.2. HPLC parameters may be changed to possibly circumvent interferences.
3.6.3. Retention time on a single column is not considered proof of chemical identity. Analyte identity should be confirmed by mass spectrometry.
3.7. Calculations
The analyte concentration for samples is obtained from the proper calibration curve in micrograms of analyte per sample. The bottom filter is analyzed to determine if there was any breakthrough from the top filter during sampling. If any analyte is found on any bottom filter, that amount is added to the amount found on the corresponding top filter. The combined amount is then corrected by subtracting the total amount (if any) found on the corresponding blank filters. The air concentrations are calculated using the following formulae.
| µg/m3 = | (µg of analyte per sample) (1000)
|
ppb = (µg/m3) (24.46) / (219.29) = (µg/m3) (0.1115)
where 24.46 is the molar volume at 25°C and 101.3 kPa (760 mmHg)
and 219.29 is the molecular weight for
3.8. Safety precautions (analytical)
- 3.8.1. CAUTION. THESE AROMATIC AMINES SHOULD BE CONSIDERED
CARCINOGENIC. Restrict the use of the pure amines and
concentrated standards to regulated areas. Avoid skin contact and
inhalation of all chemicals.
3.8.2. Restrict the use of all chemicals to a fume hood if possible.
3.8.3. Wear safety glasses and a lab coat at all times while in the lab area.
4. Backup Data
- 4.1. Detection limit of the analytical procedure
The detection limits of 180 and 32.5 pg per injection were
determined by making 10-µL injections of dilute standards
equivalent to 36.0 and 6.49 ng per sample for
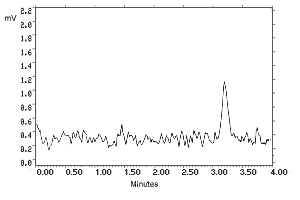 Figure 4.1.1. N-Phenyl-1-naphthylamine analytical detection limit chromatogram. |
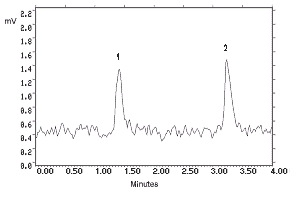 Figure 4.1.1. N-Phenyl-2-naphthylamine analytical detection limit chromatogram. Key: (1) matrix artifact, (2) |
4.2. Detection limit of the overall procedure
The detection limits of the overall procedure were determined by
analyzing filters spiked with 36.0 and 6.49 ng of
4.3. Reliable quantitation limit
The reliable quantitation limits were determined by analyzing
filters spiked with 36.0 and 6.49 ng of
|
| ||||||||||||||||||||||||||||||
4.4. Instrument response to the analyte
The instrument response to the analytes over the range of 0.5 to 2
times the target concentrations was determined from multiple
injections of analytical standards. The response is linear for both
analytes with slopes (in area counts per micrograms of analyte per
sample) of 208,500 and 1,289,000 for
|
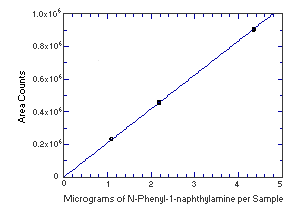 Figure 4.4.1. Instrument response to | ||||||||||||||||||||||||
|
| |||||||||||||||||||||||||
|
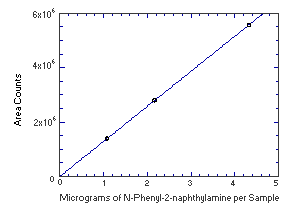 Figure 4.4.2. Instrument response to | ||||||||||||||||||||||||
4.5. Storage test
Thirty-six storage samples for each analyte were prepared by
spiking microliter amounts of intermediate standards onto Vitamin C
treated glass fiber filters. The amounts of analytes spiked (2.164
µg of
|
| |||||||
| days of storage |
% recovery (refrigerated) |
% recovery (ambient) | |||||
|
| |||||||
| 0 0 3 7 10 14 17 |
100.7 101.4 98.9 102.4 96.3 102.5 102.2 |
99.4 97.7 99.2 97.5 96.9 101.8 99.6 |
96.2 99.5 101.2 97.9 101.9 102.2 96.1 |
100.7 101.4 98.1 99.2 97.8 97.7 98.9 |
99.4 97.7 100.5 100.5 98.8 99.3 101.5 |
96.2 99.5 100.4 98.4 99.2 97.6 98.1 | |
|
| |||||||
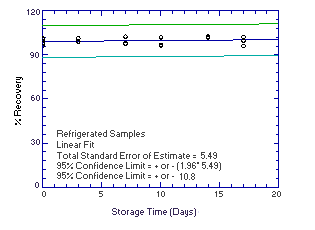 Figure 4.5.1.1. Refrigerated |
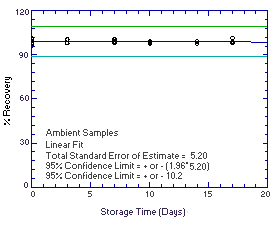 Figure 4.5.1.2. Ambient |
|
| |||||||
| days of storage |
% recovery (refrigerated) |
% recovery (ambient) | |||||
|
| |||||||
| 0 0 3 7 10 14 17 |
99.4 100.0 99.4 99.4 99.5 101.3 99.8 |
99.7 99.4 99.8 99.3 98.5 101.0 99.5 |
100.3 99.3 100.1 98.9 99.6 99.2 99.1 |
99.4 100.0 97.2 97.6 98.7 98.4 98.0 |
99.7 99.4 99.6 96.8 96.5 98.4 98.2 |
100.3 99.3 99.6 94.0 93.2 96.9 99.0 | |
|
| |||||||
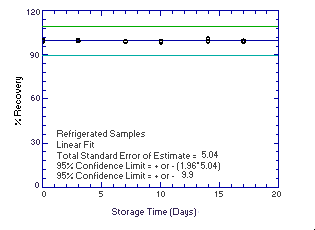 Figure 4.5.2.1. Refrigerated |
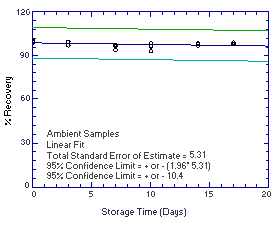 Figure 4.5.2.2. Ambient |
4.6. Precision (analytical method only)
The precision of the analytical method for each analyte is the
pooled coefficient of variation determined from replicate injections
of standards. The coefficients of variation (CV) are calculated from
the data from Tables 4.4.1. and 4.4.2. The pooled coefficients of
variation are 0.0043 and 0.0019 for
|
| ||||||||||||||||||||||||||||||||||||||||
4.7. Precision (overall procedure)
The precision of the overall procedure is determined from the storage data. The determination of the standard error of estimate (SEE) for a regression line plotted through the graphed storage data allows the inclusion of storage time as one of the factors affecting overall precision. The SEE is similar to the standard deviation, except it is a measure of dispersion of data about a regression line instead of about a mean. It is determined with the following equation:
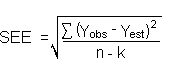
| where | n = k = k = |
total no. of data points 2 for linear regression 3 for quadratic regression |
| Yobs = | observed percent recovery at a given time | |
| Yest = | estimated percent recovery from the regression line at the same given time |
An additional 5% for pump error is added to the SEE by the addition
of variances. The precision at the 95% confidence level is obtained by
multiplying the SEE (with pump error included) by 1.96 (the
4.8. Reproducibility
Samples were prepared by injecting microliter quantities of
standards onto Vitamin C treated filters, assembling the filters into
cassettes, and drawing 240 L of 80% relative humidity air through the
samplers. The samples were stored for 11 days at 0°C before being
analyzed by another chemist. No individual sample result deviated from
its theoretical value by more than the corresponding precision of the
overall procedure. The precisions of the overall procedure are ±10.2%
and ±10.4% for
|
| |||||||||||||||||||||||||||||||||||||||||
4.9. Extraction efficiency
Six Vitamin C treated filters for each analyte were spiked with the
target concentration amounts by liquid injection (2.164 and 2.153
µg of
|
| |||||||||||||||||||||||||||||||
5. References
- 5.1. "OSHA Analytical Methods Manual", 2nd ed.;
U.S. Department of Labor, Occupational Safety and Health
Administration; OSHA Analytical Laboratory: Salt Lake City, UT, 1990;
Method 57; American Conference of Governmental Industrial Hygienists
(ACGIH): Cincinnati, OH, Publication No. 4542.
5.2. ibid. Method 65.
5.3. ibid. Method 71.
5.4. ibid. Method 73.
5.5. ibid. Method 78.
5.6. Elskamp, C.J. "OSHA Method No. 87;
5.7. Elskamp, C.J. "OSHA Method No. 93;
4-Aminobiphenyl, 1-Naphthylamine, and
5.8. "Documentation of the Threshold Limit Values and Biological Exposure Indices", 5th ed.; American Conference of Governmental Industrial Hygienists Inc.: Cincinnati, OH, 1986; p 73.
5.9. Lewis, R.J., Sr., Ed. "Sax's Dangerous Properties of Industrial Materials", 8th ed.; Van Nostrand Reinhold Co.: New York, NY, 1992.
5.10. Occupational Health Services, Inc.:
Material Safety Data Sheets (MSDS) for
5.11. "IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Overall Evaluations of Carcinogenicity:
An Updating of IARC Monographs", Volumes 1 to 42, Suppl 7, pp
5.12. Sax, N.I.; Lewis, R.J. Sr., Eds. "Hawley's Condensed Chemical Dictionary", 11th ed.; Van Nostrand Reinhold Co.: New York, NY, 1987.