- Introduction
This method describes the collection by
charcoal tube and analysis using Ion Chromatography of acetic and formic
acids. The method measures the total concentration of the airborne
anions. The corresponding acids may be collected on a single charcoal
tube and determined simultaneously.
1.1 History
Prior to the use of
this method, acetic acid was collected on charcoal tubes, desorbed in
0.1 N NaOH and analyzed by gas chromatography. Before that, acetic and
formic acids were collected in 0.01 N NaOH, and were analyzed by ion
chromatography.
1.2 Uses (9.1, 9.2)
Acetic
acid is mainly used in the manufacture of cellulose acetate fibers and
plastics, ester solvents, dyes, metal salts, and many other chemicals.
Acetic acid is one of the most important industrial organic acids. It is
most widely known in the form of vinegar, which is a dilute aqueous
solution of acetic acid.
Formic acid is important in
textile souring, leather preparation, and cattle-fodder preservation. It
is also used in the textile industry, and as an intermediate in the
production of many chemicals. It is also used as a coagulant for
rubber latex. Formic acid is used in nickel plating baths, and in the
production of wire-stripping compounds needed for soldering bare
wire.
1.3 Physical Properties (9.1, 9.2)
Acetic acid, CH3COOH, is a colorless, water-like
liquid that has a sharp, vinegary odor and a burning taste. Commercial
grades of acetic acid are approximately 99.5% pure. Acetic acid is
soluble in alcohol and water. Acetic acid is highly caustic to the
skin.
Formic acid, HCOOH, is a colorless, odorous acid. It
is the first and strongest of the unsubstituted series of carboxylic
acids. Formic acid decomposes readily to water and carbon monoxide.
Formic acid or its aqueous solutions dissolve many of the more active,
main group metals and their oxides to give corresponding formates. The
anhydrous acid must be handled with the same care as concentrated
sulfuric acid. It is a powerful dehydrating agent and serious burns can
result from its action on the skin. Formic acid burns when ignited.
Commercial grades of formic acid are approximately 90% pure. Formic acid
is soluble in water, alcohol, ether, and glycerol.
| Physical Constants: |
HCOOH |
CH3COOH |
|
|
|
| Density, 20.0ºC: |
1.220 g/cm3 |
1.049 g/mL |
|
|
|
| Melting Point: |
8.4ºC |
16.6ºC |
|
|
|
| Boiling Point: |
100.7ºC |
117.9ºC |
|
|
|
| Formula Weight: |
46.03 |
60.06 |
- Working Range and Detection Limit (9.3.)
2.1 The working range for a 48 liter air
sample is 0.002 to 0.05 ppm for HCOO¯ and
CH3COO¯. This corresponds to 0.1 to 2.5 µg. The
upper range can be extended by sample dilution.
2.2 The
quantitative detection limits for acetic and formic acids were
calculated using the IUPAC Method. The detection limits are as follows
at a confidence level of 99.86%:
HCOOH = 0.0016 ppm or 16
ng
CH3COOH = 0.006 ppm or 60 ng
The
detection limits were calculated based on a sample volume of 10 mL and
an injection volume of 50 µL. The detection limits for each analyte were
calculated in the presence of the other. The detection limits are for a
48 liter air volume at a 0.2 liters per minute sampling
rate.
- Stability of acetic and formic acids on charcoal tubes.
3.1 A recovery study of acetic and
formic acids was done at the 0.5X, 1.0×, and 2.0× levels. Six samples at
each of three levels were spiked, let sit for an hour and then desorbed
with 0.0015 M borate eluent. The samples were analyzed by ion
chromatography. The results can be seen in Table III. Two problems were
seen in the formic acid analysis. First the charcoal tubes contained an
interference which had the same retention time as formic acid. The mean
of this interference is 85.9 µg (n = 5, std. dev. = 12.2) for section A,
and 48.7 µg (n = 5, std. dev. = 3.3) for section B. The second problem
is that the formic acid is not stable unless refrigerated. See section
3.2. NIOSH found a CVT of 0.058, with a CV1 of
0.007 and a CV2 of 0.030 for their recoveries when desorbed
with formic acid and analyzed for acetic acid by gas chromatography
(9.4).
3.2 Acetic acid recovery from charcoal tubes
after 2 weeks of storage is 93% when the samples are not refrigerated
and 96% when refrigerated. Formic acid recovery is 18% for samples
stored for two weeks when not refrigerated and 82% when refrigerated.
Therefore, the samples must be kept refrigerated at 4ºC when analysis of
formic acid is desired. See Table I.
3.3 Once the samples
are desorbed in 0.0015 M borate eluent, they are stable for at least 2
weeks. Samples desorbed in the manner just described and kept
unrefrigerated for 2 weeks showed a recovery of 94% for formic acid and
90% for acetic acid.
3.4 Acetate and formate standards in
0.0015 M borate eluent were determined to be stable for at least 2
weeks.
3.5 A breakthrough study was conducted under the
following conditions.
Six charcoal tubes were spiked with
acetic and formic acids at 2.0× the PEL and then exposed to 80% humidity
air at 22ºC at a flow of rate 0.2 liters/minute for four hours. No
breakthrough was detected in the second portion of the charcoal tubes
for either acetic or formic acid. The results of the breakthrough study
can be seen in Table II. A typical chromatogram of a standard containing
1.0 µg acetate and formate is shown in Figure I.
- Interferences
4.1 Butyric and propionic acids can
cause an interference. If butyric acid is present, it can be separated
from the formic acid peak by changing the flow rate to 1.0 mL/min.
Propionic acid is difficult to separate from the acetic acid peak. The
presence of butyric or propionic acids in the workplace must be reported
to the laboratory.
- Advantages and Disadvantages
5.1 The method is accurate and easily
automated.
5.2 The sampling procedure uses charcoal tubes
as opposed to older impinger sampling methods for these acids. Such a
sampling procedure eliminates the inherent problems of spillage and
release of a caustic solution or the potential contamination of the pump
while using a impinger.
- Sampling Procedure
6.1 Apparatus - Charcoal tubes, SKC Cat.
No. 226-09 (or equivalent charcoal tubes which have been demonstrated to
show low levels of the anions of interest), personal sampling pump which
are calibrated at the recommended flow rate with a charcoal tube in line
to an accuracy of ±10% at the 95% confidence limit.
6.2
The charcoal tube is attached to a calibrated personal sampling pump and
the sampling tube is placed in the worker's breathing zone or in a area
of the workplace. At least 10 liters of air should be drawn through the
sampling tube.
6.3 After the desired sampling period is
completed, the charcoal tube is removed from the pump. The charcoal
tubes are then properly identified and official sealed with a OSHA Form
21, and shipped to the laboratory for analysis. Samples taken for formic
acid must be shipped to the laboratory via overnight mail on ice. Proper
planning should be undertaken to eliminate any possible delay in samples
arriving at the laboratory before the ice is spent.
6.4
With each batch of up to 20 samples, a blank tube which has had no air
drawn through it, is submitted for analysis. The blank tube should be
from the same lot of tubes used for sampling.
6.5 It is
very important that when particulate acids or salts of an acid are known
to be present in the workplace atmosphere each should be listed as
interferences. It is important to note the presence of other low
molecular weight carboxylic acids, particularly propionic
acid.
- Analytical Procedure
7.1 Apparatus - Ion exchange
chromatograph and recorder, or integrator (an auto sampler helps
automate the analysis), 10 mL pipette, 1 mL plastic syringe with male
Luer fitting, anion separator column with precolumn, anion membrane
suppressor, and appropriate volumetric glassware for dilutions and
standard preparation.
7.2 Reagents - All reagents used
should be ACS analyzed reagent grade or better.
7.2.1 Deionized water with a specific conductance of 10
umho/cm or less for preparation of eluents and other solutions which
will be used in the Ion Chromatograph.
7.2.2 Sodium
Borate (Na2B407·10H20).
7.2.3 Acetate Stock Standard (1000 µg/mL
CH3COO¯). Dissolve 0.6948 g of Sodium Acetate
(CH3COONa) or 1.1525 g of
CH3COONa·3H20 into 500 mL deionized water.
7.2.4 Formate Stock Standard (1000 µg/mL
HCOO¯). Dissolve 0.7554 g of Sodium Formate
(HCOONa) into 500 mL deionized water.
7.2.5 Borate
Eluent and Desorption Solution (0.0015 M
B4O7=). Dissolve 1.25 g Sodium Borate
(Na2B4O7·10H2O) in a 2
liter volumetric flask and dilute to volume with deionized water.
Sonicate the solution under a vacuum for 5 minutes before use.
7.2.6 Regenerant Solution (0.025 N
H2SO4). Add 2.8 mL of concentrated
H2SO4 to 4 liters in deionized water. Sonicate
the solution under a vacuum for 5 minutes before use. 7.3
Safety Precautions
7.3.1 When using the Ion Chromatograph, the column door
should be kept closed during the analysis in case the columns burst.
To avoid this danger the pressure should be checked at the beginning
of the analysis and periodically during the analysis.
7.3.2 Care should be used when handling reagents, especially
the regenerant solution (0.025 N H2SO4) to avoid
chemical burns.
7.3.3 Care should be exercised when
using laboratory glassware. Chipped pipettes, volumetric flasks,
beakers, or any glassware with sharp edges exposed should not be used
to avoid the possibility of cuts, abrasions, and lost samples.
7.3.4 Pipetting should never be done by mouth - a bulb should
always be used. 7.4 Standard Preparation
7.4.1 Working standards are prepared in the analytical
range of 2 µg/mL to 50 µg/mL from dilutions of the 1000 µg/mL stock
solutions. These standard solutions should be diluted to volume in
0.0015 M borate eluent and prepared fresh weekly.
7.4.2
If an auto sampler capable of variable volume injections is used, a
combination 50 µg/mL acetate and formate standard is used. This
intermediate working standard should be prepared fresh weekly.
Injection volumes should always be 50 microliters or above for
standards or samples. 7.5 Sample Preparation
7.5.1 The charcoal tube used to collect acetic and formic
acids is separated into 2 parts. The first section (section A)
contains 100 mg charcoal. The backup section (section B) contains 50
mg charcoal and will collect any acid which passes through and is not
collected by section A. Sections A and B are separated by a foam plug
which is to be discarded.
7.5.2 Score the sampler with a
file in front of the primary sorbent section (section A), then break
the sampler at the score line. Transfer section A to a clean, labeled
20 mL vial. Place charcoal section B in a separate clean, labeled 20
mL vial.
7.5.3 If the air volume is greater than or
equal to 10 liters pipette 10 mL desorption solution into each sample
vial and cap tightly. If the air volume is less than 10 liters use 5
mL desorption solution.
7.5.4 Let the samples sit
overnight in the vials, or sonicate the samples for 10 minutes. Sample
solutions must be filtered before analysis using LID/X syringe filters
from Xydex Corporation, or equivalent, and the blank should be treated
in a similar manner.
7.5.5 If using an auto sampler,
transfer some of the sample into an appropriate sampling vial. If
using the WISP autosampler, the vial should be at least half full.
Label each vial with the appropriate laboratory identification number.
If using the Dionex autosampler, place an aliquot of 0.63 mL of each
sample in separate polyvials. When using automatic injection use a 50
µL injection volume. The autosampler is less accurate below 50
µL.
7.5.6 For hand injection, use 1 mL of the eluent to
flush the 0.1 mL injection loop thoroughly. 7.6
Analysis (9.4)
7.6.1 For general instrument start up and operation, refer
to Section 8 of the Ion Chromatography Standard Operating
Procedure.
7.6.2 The normal instrument parameters
are:
Sensitivity: 30 µmho full scale
Eluent:
0.0015 M B4O7=
Flow Rate:
2.0 L/min
Run Time: Approximately 10 minutes.
7.6.3 If using the WISP autosampler, after the instrument is
set up and stabilized, place the auto sampling vials into the sampling
tray using tray positions one through five for standards.
7.6.4 If using the Dionex autosampler, refer to sections 8.1
to 8.3 of the Standard Operating Procedure.
7.6.5 Enter
the proper parameters into the auto sampler (See the Ion
Chromatography Standard Operating Procedure).
7.6.6
Start the auto sampler and observe the first few chromatograms to
ensure proper operation. Periodically check the zero offset between
samples to correct any baseline drift and to ensure proper sensitivity
and retention time of the analytes.
7.6.7 Use the timer
to stop the run if the auto sampler is to be left unattended.
7.6.8 Record the sample number onto the chromatogram. Keep a
record of the sample identity and instrument conditions.
7.6.9 As the analysis proceeds, check the retention times of
standards vs. samples to ensure uniformity.
7.6.10 If
interfering substances are present, establish positive identity of the
peaks by spiking known amounts of standard solution or try to obtain
better separation by changing the eluent concentration or by reducing
the flow rate. 7.7 Calculations
7.7.1 Peak areas or heights of the standards are used to
construct a standard curve using the OSHA Auto Colorimetric Program.
The samples results are obtained from a plot of peak height or peak
area vs. concentration. The blank corrected sample values are then
calculated using the Auto Colorimetric Program.
7.7.2
When using the OSHA Auto Colorimetric Program, sample numbers and
volumes are entered into the calculator in the following manner:
Sample Number, Peak Area or Height, L Air Volume, mL Solution
Volume, mL Aliquot Volume.
7.7.3 Air Concentration
values are calculated by the following equation:
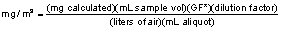
GF* = Gravimetric Factor = 1.02 for
HCOOH and CH3COOH
7.7.4 Acetic and formic acids are
reported in ppm. To convert the mg/m3 values to ppm, the
mg/m3 value must be multiplied by the appropriate
conversion factor.
| Acid |
Conversion
Factor |
|
|
| HCOOH |
0.532 |
|
|
| CH3COOH |
0.407 |
- Reporting Results for Compounds Determined by Ion
Chromatography
8.1 Results are reported on the OSHA
Form 91 in ppm for HCOOH and CH3COOH, using two significant
figures.
8.2 The estimated detection limit calculated by
the Auto Colorimetric Program is reported on the OSHA Form 91 when no
analyte is detected.
8.3 The presence of significant
unidentifiable peaks is noted on the OSHA Form 91.
8.4 All
data processor printouts and chart recorded chromatograms are filed in a
central file according to laboratory sample identification.
8.5 Calculations are checked by a fellow chemist before the
completed OSHA Form 91's are given to the supervisor.
- References
9.1 Encyclopedia of
Chemical Technology, Third Edition, 1978, Vol. 1 and 11.
9.2 Merck Index, Tenth Edition,
1983.
9.3 OSHA Ion Chromatography Standard Operating
Procedure, Prepared by the Ion Chromatography Committee, Occupational
Safety & Health Administration Analytical Laboratory, Inorganic
Division.
9.4 Backup Data Report for Acetic Acid Method
(by GC), NIOSH, Cincinnati, OH.
TABLE
I
STABILITY STUDY |
|
|
Refrigerated |
|
|
| -----------
Acetic Acid----------- |
-----------
Formic Acid----------- |
| Day 0 |
Day 7 |
Day 14 |
Day 0 |
Day 7 |
Day 14 |
| 1.034 |
1.051 |
0.964 |
1.149 |
0.999 |
0.821 |
|
|
Unrefrigerated |
|
|
| -----------
Acetic Acid----------- |
-----------
Formic Acid----------- |
| Day 0 |
Day 7 |
Day 14 |
Day 0 |
Day 7 |
Day 14 |
| 1.034 |
1.026 |
0.931 |
1.149 |
0.285 |
0.182 |
TABLE
II
BREAKTHROUGH STUDY
|
| Sample # |
Recovery Acetic
Acid |
Recovery Formic
Acid |
| 1 |
0.777 |
0.700 |
| 2 |
0.764 |
0.723 |
| 3 |
0.787 |
0.691 |
| 4 |
0.774 |
0.716 |
| 5 |
0.782 |
0.714 |
| 6 |
0.752 |
0.695 |
| Mean |
0.773 |
0.706 |
| Std Dev |
0.013 |
0.013 |
TABLE
III
RECOVERY STUDY
Acetic
Acid
Found/Theoretical
|
| Sample # |
0.5× PEL |
1.0× PEL |
2.0× PEL |
| 1 |
0.924 |
0.878 |
1.071 |
| 2 |
1.177 |
0.951 |
1.080 |
| 3 |
0.999 |
0.951 |
0.980 |
| 4 |
0.986 |
0.759 |
0.982 |
| 5 |
0.921 |
0.784 |
1.005 |
| 6 |
0.940 |
0.850 |
0.999 |
| n = |
6 |
6 |
6 |
| Mean = |
0.991 |
0.862 |
1.020 |
| Std Dev = |
0.097 |
0.081 |
0.045 |
| CV1
= |
0.098 |
0.094 |
0.044 |
| CV1
(pooled) |
|
0.0068 |
|
Formic
Acid
Found/Theoretical
|
| Sample # |
0.5× PEL |
1.0× PEL |
2.0× PEL |
| 1 |
1.108 |
0.966 |
1.069 |
| 2 |
1.338 |
1.032 |
0.982 |
| 3 |
1.159 |
0.994 |
0.920 |
| 4 |
1.272 |
0.792 |
0.931 |
| 5 |
1.032 |
0.855 |
1.009 |
| 6 |
1.334 |
0.920 |
0.972 |
| n = |
6 |
6 |
6 |
| Mean = |
1.207 |
0.926 |
0.980 |
| Std Dev = |
0.127 |
0.090 |
0.054 |
| CV1
= |
0.105 |
0.097 |
0.055 |
| CV1
(pooled) |
|
0.0078 |
|
|

