1. General Discussion
1.1 Background
1.1.1 History of procedure
The OSHA Laboratory recently received samples collected
on silica gel requesting analysis for p-chloroaniline. Several
analytical procedures were tried, including gas chromatography and
liquid chromatography, but the sensitivity of the liquid
chromatography method was greater, and therefore it was used. The
liquid chromatograph had an ultraviolet detector set at 254 nm, a
secondary wavelength of 280 nm can also be used. The samples were
desorbed with methanol and the desorption efficiency was 100%.
1.1.2 Potential workplace exposure
Workers are
exposed to p-chloroaniline in many industries, with the majority of
them in the chemical manufacturing industries. It is used in azoic
coupling compounds and dye manufacturing (Ref. 5.1).
1.1.3 Toxic Effects (This section is included for information
only and should not be taken as a basis of OSHA policy.)
Exposure to p-chloroaniline is irritating to skin, eyes,
mucous membranes, and through oral ingestion and inhalation. Upon
entering the bloodstream p-chloroaniline damages the blood cells. The
lowest toxic concentration by inhalation in humans was 44
mg/m3(8.4 ppm). The lowest lethal dose through oral
ingestion in rats was 300 mg/kg. The lowest lethal dose applied
dermally in rabbits was 36 mg/kg (Ref. 5.2).
1.1.4
Physical properties: (Ref. 5.3)
| Molecular weight:
127.57 |
| Melting point:
72.5°C |
| Boiling point:
232°C |
| Odor:
aniline-like |
| Color: white to pale
gray |
| Vapor pressure: 1 mm
at 59.3°C |
| Molecular formula:
ClC6H4NH2 |
| Structure: |

1.2 Limit defining parameters
1.2.1 The detection limit of the
analytical procedure is 6 ng/injection. This is the smallest amount
that could be easily detected under normal analytical conditions.
The injection size was 10 µL.
1.2.2 The detection
limit of the overall procedure is 6 ng/sample (0.02 ppm based on a
6-liter sample). This is the amount of analyte placed on a silica gel
tube which corresponds to the detection limit of the analytical
procedure.
1.3
Advantages
1.3.1 The sampling procedure is
convenient.
1.3.2 The analytical method is reproducible
and
sensitive.
1.3.3 Re-analysis of samples is
possible.
1.3.4 It may be possible to analyze other
compounds
at the same time.
1.3.5 Interferences
may be avoided by proper selection of column and
parameters.
1.4
Disadvantages
1.4.1 Silica gel tubes adsorb
water vapor from, the air, thereby possibly lowering the capacity of
the tube for p-chloroaniline.
2. Sampling
procedure
2.1 Apparatus
2.1.1 A calibrated personal
sampling pump, the flow
of which can be determined within +
5% at the recommended flow.
2.1.2 Silica gel tubes
(20/40 mesh) containing 150-mg adsorbing section with a 75-mg backup
section separated by a 2 mm portion of urethane foam, with a
silane-treated glass wool plug before the adsorbing section and a 3-mm
plug of urethane foam at the back of the backup section. The ends are
flame sealed and the glass tube containing the adsorbent is 7-mm long,
with a 6-mm O.D. and 4-mm I.D., SKC tubes or equivalent.
2.2 Sampling technique
2.2.1 The ends of the silica gel
tube are opened immediately before sampling.
2.2.2
Connect the silica gel tube to the sampling pump with flexible tubing.
2.2.3 Tubes should be placed in a vertical position to
minimize channeling, with the smaller section towards the pump.
2.2.4 Air being sampled should not pass through any hose or
tubing before entering the silica gel tube.
2.2.5 Seal
the silica gel tube with plastic caps immediately after sampling. Seal
each sample lengthwise with OSHA Form-21 sealing tape.
2.2.6 With each batch of samples, submit at least
one
blank tube from the same lot used for samples. This tube
should be subjected to exactly the same handling as the samples (break
ends, seal, & transport) except that no air is drawn through
it.
2.2.7 Transport the samples (and corresponding
paperwork) to the lab for analysis.
2.2.8 Bulks
submitted for analysis must be shipped in a separate container from
the samples.
2.3
Desorption efficiency
2.3.1 The desorption efficiency
averaged 100% at the concentration of 149.2 µg loading on the tubes,
or 4.77 ppm based on a 6 liter air sample (see Table 2.3.2).
2.3.2 The desorption study was generated by spiking 298.3,
149.2, or 74.59 µg of p-chloroaniline onto six tubes at each level and
storing overnight at room temperature. They were opened the next day,
desorbed with 1-mL methanol, and analyzed by LC-UV.
Table 2.3.2
| Level spiked (µg) |
%Recovered |
| 298.3 |
98.7 |
| 298.3 |
102 |
| 298.3 |
102 |
| 298.3 |
102 |
| 298.3 |
99.7 |
| 298.3 |
99.8 |
| average |
101 |
| |
|
| 149.2 |
101 |
| 149.2 |
101 |
| 149.2 |
99.8 |
| 149.2 |
102 |
| 149.2 |
101 |
| 149.2 |
102 |
| average |
101 |
| |
|
| 74.59 |
99.1 |
| 74.59 |
98.4 |
| 74.59 |
101 |
| 74.59 |
101 |
| 74.59 |
102 |
| 74.59 |
104 |
| average |
101 |
| overall average |
101 |
| standard deviation |
±1.44 |
2.4 Retention efficiency
2.4.1 The retention efficiency was
performed by liquid spiking silica gel tubes with 149.2 µg
p-chloroaniline and drawing a known volume of humid air (R.H. 86%)
through the tubes. There was little loss of the p--chloroaniline (see
Table 2.4.2).
2.4.2 Twelve samples were spiked with 149.2 µg
p-chloroaniline. Six had 3 liters, and the other six samples had 6
liters of humid air drawn through them. There was 1.6% loss with 3
liters and 2.9% loss with 6 liters humid air
drawn.
Table 2.4.2
| Tube # |
Liters drawn |
% recovered |
| 1 |
3 |
98.0 |
| 2 |
3 |
98.8 |
| 3 |
3 |
99.7 |
| 4 |
3 |
99.3 |
| 5 |
3 |
96.4 |
| 6 |
3 |
96.4 |
| average |
3 |
98.4 |
| |
|
|
| 7 |
6 |
98.2 |
| 8 |
6 |
96.0 |
| 9 |
6 |
98.2 |
| 10 |
6 |
98.1 |
| 11 |
6 |
95.0 |
| 12 |
6 |
lost in
analysis |
| average |
6 |
97.1 |
2.5. Storage
2.5.1 Storage study was performed over
a 14-day period with little loss of p-chloroaniline (see Table
2.5.2).
2.5.2 The tubes were spiked with 149.2 µg/tube.
They were analyzed on days 1, 6, 11, and 14. The day I samples were
used for the desorption study.
Table 2.5.2
| Day |
% Recovered |
| 1 |
101 |
| 1 |
101 |
| 1 |
99.8 |
| 1 |
102 |
| 1 |
101 |
| 1 |
102 |
| 6 |
96.5 |
| 6 |
97.3 |
| 6 |
95.6 |
| 11 |
99.6 |
| 11 |
97.9 |
| 11 |
99.8 |
| 14 |
97.5 |
| 14 |
97.3 |
| 14 |
100 |
2.6.Air volume and sampling rate studied
2.6.1 The air volume studied is 6
liters.
2.6.2 The sampling rate studied is 0.1 liters
per minute.
2.7
Interferences
2.7.1 Water vapor may cause a
decrease in the collection capacity of p-chloroaniline.
2.7.2 Suspected interferences should be listed on sample data
sheets.
2.8 Safety
precautions
2.8.1 Sampling equipment should be
placed on an employee in a manner that does not interfere with work
performance or safety.
2.8.2 Safety glasses should be
worn at all times.
2.8.3 Follow all safety practices
that apply to the workplace being sampled.
3. Analytical method
3.1 Apparatus
3.1.1 Liquid chromatograph
equipped with an ultraviolet detector at 254 rim.
3.1.2 LC column capable of separating the analyte from any
interferences. An Alltech C18 column was used in this evaluation.
3.1.3 An electronic integrator or some other suitable method
of measuring peak areas.
3.1.4 Two milliliter vials with
Teflon-lined caps.
3.1.5 A 10-µL syringe or other
convenient size for sample injection.
3.1.6 Pipets for
dispensing the desorbing solvent. The Glenco 1-mL dispenser was used
in this method.
3.1.7 Volumetric flasks 10 mL and other
convenient sizes for preparing standards.
3.2 Reagents
3.2.1 Methanol HPLC grade.
3.2.2 Millipore deionized, water.
3.3 Sample preparation
3.3.1 Sample tubes are opened and
the front and back section of each tube are placed in separate
2-mL vials.
3.3.2 Each section is desorbed with 1 mL
methanol.
3.3.3 The vials are sealed immediately and allowed
to desorb for 30 minutes with occasional shaking.
3.4 Standard
preparation
3.4.1 Standards are prepared by
diluting a known quantity of p-chloroaniline with methanol..
3.4.2 At least two separate stock standards should be made.
Dilutions of these stock standards are made. The range used in this
method was from 1492 ug/mL to 0.6 ug/mL.
3.5 Analysis
3.5.1 Liquid chromatograph
conditions
| Solvent
mixture: |
65% methanol 35%
water |
| Injection size: |
10 µL |
| Elution time: |
6.62 minutes |
| Wavelength: |
254 nm |
| Flow rate: |
1mL/min |
| Column: |
Alltech
C18 |
| Chromatogram: |
(See Figure
1) |
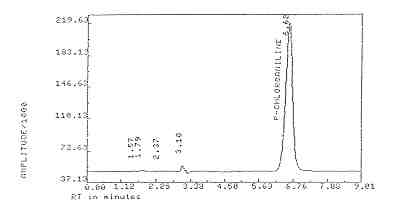
3.5.2. Peak areas are measured
by an integrator or other suitable means.
3.6. Interferences (analytical)
3.6.1. Any compound having the
general retention time of the analyte is an interference.
Possible interferences should be listed on the sample data sheet. LC
parameters should be adjusted if necessary so these interferences will
pose no problems.
3.6.2. Retention time data on a single
column is not considered proof of chemical identity. Samples over the
target concentration should be confirmed by GC/Mass Spec or other
suitable means.
3.7.
Calculations
3.7.1 To calculate the ppm of
analyte in standards based on a 6 liter air sample, and a 1-mL
desorbing solution:
| 24.46 |
= Molar volume
(liters/mole) at 25°C and 760 mmHg. |
| MW |
= Molecular
weight |
| p |
= Density |
| 1 mL |
= Desorption rate |
| 6 L |
= 6 liter air
sample |
| DE |
= Desorption
efficiency |

3.7.2 This calculation
is done for each section of the sampling tube and the results added
together.
3.8.Safety
precautions
3.8.1 All handling of solvents
should be done in a hood.
3.8.2 Avoid skin contact with
all solvents.
3.8.3 Wear safety glasses at all
times.
4.
Recommendations for further study vapor generated samples in both dry and
humid air should be performed to check adsorption of p-chloroaniline on
silica gel tubes. Since there may be a problem with water vapor replacing
the p-chloroaniline on the silica gel tubes, the retention efficiency at
larger air volumes of humid air needs to be
studied.
5.
References
5.1 Mark, H.F., Othmer, D.F.,
Overberger, C.G., Seaborg, G.T., "Encyclopedia of Chemical Technology",
Third Edition, John Wiley & Son, N.Y., 1978, Vol. 2, p. 318.
5.2 Sax, N.I., "Dangerous Properties of Industrial materials",
Fifth Edition, Van Nostrand Reinhold Co., N.Y., 1979, p.488.
5.3 Windholz, M., "The Merck Index", Tenth Edition, Merck &
Co., Rahway N.J., 1983, p.297.
|

