1. General Discussion
1.1 Background
1.1.1 History of procedure
The
OSHA Analytical Laboratory received a set of field samples requesting
the analysis of norethindrone and mestranol. These air samples had
been collected on FWS-B filters. This report describes the analytical
procedure developed for the analysis of norethindrone.
1.1.2
Toxic effects (This section is for information only and should not be
taken as the basis of OSHA policy).
There is limited evidence
for the carcinogenicity of norethindrone in animals. (Ref.
5.1)
1.1.3 Potential workplace exposure
No estimate of
worker exposure to norethindrone could be found. Norethindrone is not
produced in the United States. (Ref. 5.3)
1.1.4 Physical
properties (Ref. 5.1 and 5.2)
| Molecular weight: |
298.4 |
| Molecular formula: |
C20H26O2 |
| CAS #: |
68-22-4 |
| Melting point: |
203-204°C |
| Solubility: |
Soluble in ethanol, acetone,
chloroform, pyridine, and dioxane. |
| Synonyms: |
17-hydroxy-19-norpregn-4-en-20-yn-3-one;
anhydroxynorprogesterone; 19-norethisterone; norpregneninolone;
Conceplan; Mini-Pe; Noriday; Anovule |
| Structure: |
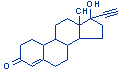 |
| Description: |
White crystalline powder with a
slightly bitter taste. |
| UV scan: |
| 1.2 Limit
defining parameters
The detection limit of the analytical
procedure is 1.9 ng per injection. This is the amount of analyte which
will give a peak whose height is approximately five times the baseline
noise. 2. Sampling
procedure
2.1 Apparatus
2.1.1 Samples are collected by using a
personal sampling pump that can be calibrated to within ±5% of the
recommended flow rate with the sampling device in line.
2.1.2
Mine Safety Appliances (MSA) membrane filter type FWS-B (PVC) 5.0
micron 37-mm diameter, or equivalent.
2.1.3 Backup pad, 37-mm
diameter, Millipore AP10, MF support pad, or equivalent.
2.1.4
Filter holder, 37-mm polystyrene cassette, Millipore M000037AO, or
equivalent. 2.2
Reagents
None
2.3 Sampling technique
2.3.1 Assemble the filter in the
two-piece cassette holder and close firmly. The filter is supported by
the backup pad. Secure the cassette holder together with
tape.
2.3.2 Attach the outlet of the filter cassette to the
personal sampling pump inlet with flexible tubing. Air being sampled
should not pass through any hose or tubing prior to entering the
sampler.
2.3.3 Attach the sampler vertically in the employee's
breathing zone in such a manner that it does not impede work
performance.
2.3.4 After sampling for the appropriate time,
remove the sampling device and reinstall the end-plugs on the
cassettes.
2.3.5 Wrap each sample end-to-end with an OSHA seal
(Form 21).
2.3.6 Submit at least one blank for each set of
samples. The blank should be handled in the same manner as the
samples, except no air is drawn through it.
2.3.7 Record the
air volume (in liters of air) for each sample, and list any possible
interferences.
2.3.8 Bulk samples submitted for analysis must
be sent in a separate container from air samples.
2.4. Extraction
efficiency
Twelve FWS-B filters were each spiked with 15.5
µg of norethindrone along with mestranol. Three of the filters
were extracted in 3 ml of isopropanol by shaking for 30 min. and
then analyzed. The results are listed in table 2.4.
2.5
Retention efficiency
To the remaining nine filters from
above, 500 L of humid air (80% relative humidity) was drawn
through each filter. Three of the filters were extracted with 3 mL
of isopropanol by shaking for 30 min. and then analyzed. The
results are listed in table 2.5.
2.6 Sample
storage
The remaining six samples from above were stored.
Three of the samples were stored at ambient temperature in a
drawer, and three were stored in a refrigerator. After six days of
storage, the samples were extracted with 3 mL of isopropanol by
shaking for 30 min. and then analyzed. The results are given in
the tables below. |
Table
2.4
Extraction Efficiency
|
amount
spiked, µg |
amount
found,
µg |
recovered,
%
|
|
15.5
15.5
15.5
|
15.6
15.1
15.2
 |
100.6
97.4
98.1
98.7
|
Table
2.5
Retention Efficiency
|
amount
spiked, µg |
amount
found,
µg |
recovered,
%
|
|
15.5
15.5
15.5
|
15.3
15.4
15.3
 |
98.7
99.4
98.7
98.9
| |
Table 2.6.1
Ambient
Storage
|
|
Table
2.6.2
Refrigerated Storage
|
amount
spiked, µg |
amount found,
µg |
recovered,
% |
|
amount
spiked, µg |
amount found,
µg |
recovered,
% |
|
|
|
15.5
15.5
15.5 |
15.1
15.1
15.1
 |
97.4
97.4
97.4
97.4 |
|
15.5
15.5
15.5 |
15.1
15.2
15.2
 |
97.4
98.1
98.1
97.9 |
2.7 Recommended air volume and sampling rate
2.7.1 The recommended air volume is
500 L.
2.7.2 The recommended flow rate is 2.0 L/min.
2.8 Interferences
It is not
known if any compounds will interfere with the collection of
norethindrone.
2.9 Safety precautions
2.9.1 Attach the sampling equipment in
such a manner that it will not interfere with work performance or
employee safety.
2.9.2 Follow all safety practices that apply
to the work area being sampled. 3. Analytical procedure
3.1 Apparatus
3.1.1 A balance capable of weighing to
the nearest tenth of a milligram. A Mettler HL52 balance was used in
this evaluation.
3.1.2 Mechanical shaker.
3.1.3 A high
performance liquid chromatograph (HPLC) equipped with an ultraviolet
(UV) detector, a manual or an automatic injector, and a strip chart
recorder. A Waters system that included a WISP autosampler, model
6000-A pump, and a model 440 fixed wavelength detector was used in
this evaluation.
3.1.4 HPLC column capable of separating
norethindrone from any interferences. A (25 cm × 4.6 mm i.d.) Alltech
C18 (10 micron) column was used in this evaluation.
3.1.5 An
electronic integrator, or some other suitable method for measuring
detector response. The Hewlett-Packard 3357 Laboratory Data System was
used in this evaluation.
3.1.6 Vials, 4-mL with Teflon-lined
septum.
3.1.7 Volumetric flasks and pipets.
3.2 Reagents
3.2.1 HPLC grade acetonitrile
(ACN).
3.2.2 HPLC grade water. A Millipore Milli-Q system was
used to prepare the water for this evaluation.
3.2.3 HPLC grade
isopropanol.
3.2.4 Norethindrone, Sigma Chemical Company, St
Louis, MO. 3.3 Standard
preparation
Stock standards were prepared by weighing 15 mg of
norethindrone, placing in 25-mL volumetric flasks, and diluting to
volume with isopropanol. Dilutions of the stock standards were made by
pipet to obtain working range standards. Stock and dilute standards were
stored in a freezer.
3.4 Sample preparation
3.4.1 Transfer the FWS-B filters to
separate 4-ml WISP vials.
3.4.2 Pipet 3.0 mL of isopropanol
into each vial and seal with a Teflon-lined septum.
3.4.3 Shake
the vials for 30 minutes. 3.5
Analysis
3.5.1 Instrument conditions
| Column: |
Alltech C18 10-Fm, (25 cm ×
4.6 mm i.d.) |
| Eluent: |
ACN/H2O 70:30 |
| Flow rate: |
1 mL/min |
| Detector: |
Ultraviolet detector (Waters
440)
Wavelength = 254 nm |
| Retention time: |
6.4 min |
| Injection volume: |
20
µL/injection |
3.5.2
Chromatogram:
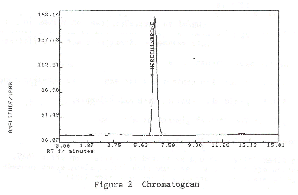
3.6 Interferences
3.6.1 Any collected compound having a
similar retention time and absorbs at 254 nm is an
interference.
3.6.2 HPLC conditions may be varied to circumvent
an interference.
3.6.3 Retention time alone is not proof of
chemical identity. Analysis by other means should be sought whenever
possible for confirmation of identity. 3.7 Calculations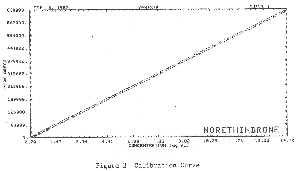
3.7.1 A calibration curve is
constructed by plotting detector response versus standard
concentration.
3.7.2 The concentration of norethindrone in a
sample is determined from the calibration curve.
3.7.3 The air
concentration is then determined by the following
formula.
mg
m³ |
= |
(µg/mL in sample)(extraction volume in mL)
(air volume in liters)(extraction
efficiency) | 3.8 Safety precautions
3.8.1 Avoid skin contact and air
exposure to norethindrone.
3.8.2 Avoid skin contact with all
solvents.
3.8.3 Wear safety glasses at all times.
4. Recommendations for
further study
Glass fiber filters should be tested for extraction
efficiency, retention efficiency, and storage stability. Also, other
procedures should be examined to obtain a lower detection limit.
5.
References
5.1 IARC Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Volume 21, International
Agency for Research on Cancer, Lyon, 1979, pp. 441-460.
5.2 Merck
Index, Tenth Edition, Merck & Co., Inc, Rahway, N.J.
1983.
5.3 IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans, Volume 6, International Agency for Research
on Cancer, Lyon, 1974, pp. 179-189. |

