1. General Discussion
1.1 Background
1.1.1 History of procedure The OSHA
Analytical Laboratory received a set of air samples requesting the
analysis of rhodamine B. The samples had been collected on polyvinyl
chloride filters (FWS-B) with air volumes around 970 liters. FWS-B
filters and glass fiber filters were both evaluated since glass fiber
filters are more commonly used. This report describes the analytical
method developed for glass fiber filters.
1.1.2. Toxic effects
(This section is for information only and should not be taken as the
basis of OSHA policy.) Rhodamine B has been tested in mice and rats by
subcutaneous injection and, in inadequate studies, by oral
administration. It was carcinogenic in rats when injected
subcutaneously, producing local sarcomas. The intravenous
LD50 in rats is 89.5 mg/kg. (Ref. 5.1)
1.1.3
Potential workplace exposure The following information is taken from
the IRAC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. (Ref. 5.1)
Rhodamine B can be used to dye silk, cotton, wool, bast fibers, nylon,
acetate fibers, paper, spirit inks and lacquers, soap, wood stains,
feathers, leather and distempers on china clay. In the U. S., it has
been used as a drug and cosmetic colour in aqueous drug solutions,
tablets, capsules, toothpaste, soap, hair-waving fluids, bath salts,
lipsticks and rouges. This color has also been used as a tracing agent
in water pollution studies, as a dye for waxes and antifreeze and as
an analytical reagent for antimony, bismuth, cobalt, niobium, gold,
manganese, mercury, molybdenum, tantalum, thallium and
tungsten.
No estimate of worker exposure to rhodamine B could
be found.
1.1.4 Physical properties (Ref. 5.1 to
5.3)
| Molecular weight: |
479 |
| Molecular formula: |
C28H31ClN2O3
|
| CAS number: |
81-88-9 |
| IMIS number: |
0848 |
| Melting point: |
165°C |
| Solubility: |
very soluble in water and alcohol;
slightly soluble in hydrochloric acid and sodium hydroxide |
| Chemical name: |
N-[9-(2-carboxyphenyl)-6-(diethylamino)
-3H-xanthen-3-ylidene]-N-ethylethanaminium
chloride |
| Synonyms: |
tetraethylrhodamine; D & C Red
No. 19; rhodamine B chloride; C.I. Basic Violet 10; C.I.
45170 |
| Description: |
green crystals or reddish-violet
powder
|
| Structure: |
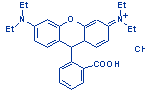 |
|
| UV data: |
546 nm maximum
(Ref. 5.1)
556
nm maximum |
|
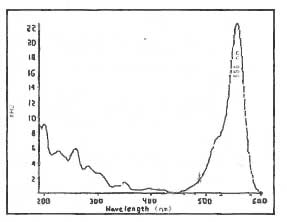
Figure 1. UV
Scan of Rhodamine B in Mobile Phase
|
1.2 Limit defining
parameters
The detection limit of the analytical procedure
is 0.013 ng per injection with a fluorescence detector or 0.42 ng
per injection with a UV detector. This is the amount of analyte
which will give a peak whose height is approximately five times
the baseline noise. (Figure 2) |
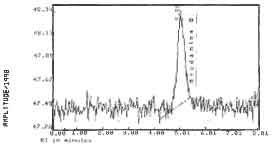
Figure
2.
Detection Limit Chromatogram of Rhodamine B on a
Fluorescence Detector | 2. Sampling procedure
2.1 Apparatus
2.1.1 A personal sampling pump that
can be calibrated to within ± 5% of the recommended flow rate with the
sampling device in line.
2.1.2 Glass fiber filters, 37-mm
diameter, Gelman Type A or equivalent.
2.1.3 Cassette filter
holders for 37-mm filters, Millipore M000037A0 or
equivalent. 2.2
Reagents
No sampling reagents are required.
2.3 Sampling
technique
2.3.1 Immediately before sampling,
remove the plastic plugs from the cassette.
2.3.2 Attach the
cassette to the sampling pump with flexible tubing.
2.3.3
Attach the cassette vertically in the employee's breathing zone in
such a manner that it does not impede work performance.
2.3.4
After sampling for the appropriate time, remove the cassette and
replace the plastic plugs.
2.3.5 Wrap each cassette end-to-end
with an OSHA seal (Form 21).
2.3.6 Record the air volume for
each sample, and list any possible interferences.
2.3.7 Submit
at least one blank for each set of samples. Handle the blank in the
same manner as the samples, except no air is drawn through
it.
2.3.8 Submit bulk samples for analysis in a separate
container. Do not ship them with air samples.
2.4 Extraction
efficiency
Six glass fiber filters were each liquid spiked
with 6 µL of a 2.21 mg/mL rhodamine B standard. After drying,
these filters were each extracted with 5.0 mL of methanol, shaken
for 30 min and then analyzed as in Section 3. The results are
listed in Table 2.4. |
Table
2.4
Desorption Efficiency
|
amount spiked,
µg |
amount found,
µg |
% recovered |
|
13.26
13.26
13.26
13.26
13.26
13.26 |
14.24
13.47
13.26
13.19
13.28
13.42 |
107.4
101.6
100.0
99.5
100.2
101.2 |
| |
 |
101.6 |
| |
2.5 Retention
efficiency
Eighteen glass fiber filters were each liquid
spiked with 6 µL of a 2.21 mg/mL rhodamine B standard. These
filters were dried and then 240 L of humid air (~80% relative
humidity) were drawn though each filter at approximately 1.3
L/min. Six of the filters were then each extracted with 5.0 mL of
methanol, shaken for 30 min and analyzed as in Section 3. The
results are listed in Table 2.5. The rest of the filters were
kept, 6 in a drawer at ambient temperature and 6 in a
refrigerator, for storage studies. |
Table
2.5
Retention Efficiency
|
amount spiked,
µg |
amount found,
µg |
% recovered |
|
13.26
13.26
13.26
13.26
13.26
13.26 |
12.23
12.59
11.88
12.33
11.58
11.37 |
92.2
94.9
89.6
93.0
87.3
85.7 |
|
 |
90.4 |
| |
2.6 Sample storage
After 2 days of storage 6
samples, 3 from ambient storage and 3 from refrigerator storage, were
each extracted with 5.0 mL of methanol, shaken for 30 min and then
analyzed as in Section 3. After 7 days of storage, the remaining samples
were extracted and analyzed. The results are given in Tables 2.6.1 and
2.6.2.
Table
2.6.1
Ambient Storage
|
days
stored |
amount
spiked,
µg |
amount
found,
µg |
%
recovered |
|
2
2
2
7
7
7 |
13.26
13.26
13.26
13.26
13.26
13.26 |
10.71
11.14
10.68
12.38
12.06
11.64 |
80.8
84.0
80.5
93.3
91.0
87.7 |
|
|
 of
2 of
2
 of 7 of 7 |
81.8
90.7 |
| |
Table
2.6.2
Refrigerator Storage
|
days
stored |
amount
spiked,
µg |
amount
found,
µg |
%
recovered |
|
2
2
2
7
7
7 |
13.26
13.26
13.26
13.26
13.26
13.26 |
11.89
11.91
12.13
11.44
11.89
12.07 |
89.6
89.8
91.4
86.3
89.7
91.0 |
|
|
 of
2 of
2
 of 7 of 7 |
90.3
89.0 |
| |
2.7 Recommended air volume and sampling rate
2.7.1 The recommended air volume is
240 L.
2.7.2 The recommended flow rate is 1.0
L/min.
2.8 Interferences
(sampling)
It is not known if any compounds will interfere with
the collection of rhodamine B. Any suspected interferences should be
reported to the laboratory.
2.9 Safety precautions
(sampling)
2.9.1 Attach the cassette in such a
manner that it will not impede work performance or employee
safety.
2.9.2 Follow all safety practices that apply to the
work area being sampled. 3. Analytical procedure
3.1 Apparatus
3.1.1 A balance capable of weighing to
the nearest tenth of a milligram. A Mettler HL52 balance was used in
this evaluation.
3.1.2 A mechanical shaker.
3.1.3 An
HPLC with UV and fluorescence detectors. A Hewlett Packard 1090 liquid
chromatograph with a diode array detector and an ABI Analytical (980G)
fluorescence detector were used in this evaluation.
3.1.4 An
HPLC column capable of separating rhodamine-B from any interferences.
A 100 mm × 2.1 mm i.d. Hypersil ODS, 5 µm, column was used in this
evaluation.
3.1.5 An electronic integrator or some other
suitable means for measuring detector response. The Hewlett-Packard
(HP) 1090 Chem Station and HP 3357 data system were used in this
evaluation.
3.1.6 Volumetric flasks and pipets of various
sizes.
3.1.7 Scintillation vials, 20-mL.
3.1.8 Vials,
2-mL. 3.2 Reagents
3.2.1 Methanol, HPLC
grade.
3.2.2 Water, HPLC grade.
3.2.3 Acetonitrile, HPLC
grade.
3.2.4 Phosphoric acid (H3PO4),
reagent grade.
3.2.5 Rhodamine B. An Aldrich standard of 99%
purity was used in this evaluation. 3.3 Standard preparation
Prepare rhodamine B stock
standards by weighing 10 to 15 mg of rhodamine B. Transfer the rhodamine
B to separate 10-mL volumetric flasks and add methanol to the mark. Make
working range standards of 0.01 to 5.0 µg/mL by pipet dilutions of the
stock standards with methanol. Store stock and dilute standards in a
freezer.
3.4 Sample preparation
3.4.1 Transfer the glass fiber filter
to a scintillation vial.
3.4.2 Add 5.0 mL of methanol to each
vial and seal with a Teflon-lined cap.
3.4.3 Shake the vials
for 30 minutes on a mechanical shaker.
3.4.4 Transfer the
sample to a 2-mL vial for use in an HP autosampler.
3.5 Analysis
3.5.1 Instrument conditions
| Column: |
100 mm × 2.1 mm i.d. Hypersil ODS, 5 µm |
| Oven temperature: |
40°C |
Mobile phase:
|
85% acetonitrile 15% water with 0.005M
1-heptanesulfonic acid and the pH adjusted to 3.5 with
H3PO4 |
| Flow: |
0.2 mL/min |
| Wavelength: |
556 nm |
Fluorescence detector:
|
Excitation: 210 nm
Emission: 550 nm cut off
filter |
| Injection volume: |
1.0 µL |
| Retention time: |
4.5 min |
3.6 Interferences
(analytical)
3.6.1 Any collected compound
having a retention time similar to that of rhodamine B is an
interference.
3.6.2 Generally, HPLC conditions may be
varied to circumvent interferences.
3.6.3 Retention time
on a single column is not proof of chemical identity. Analysis
by an alternate HPLC column, comparison of detector responses or
confirmation by mass spectrometry are additional means of
identification. |
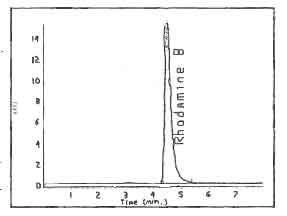
Figure
3.
Chromatogram of Rhodamine B on a UV
Detector |
3.7 Calculations
3.7.1 Construct a calibration
curve by plotting detector response versus concentration (µg/mL)
of rhodamine B.
3.7.2 Determine the µg/mL of rhodamine B
in each sample and blank from the calibration
curve.
3.7.3 Blank correct, if necessary, the sample by
subtracting the µg/mL found in the blank from the µg/mL found in
the sample.
3.7.4 Determine the air concentration by
using the following formula. |
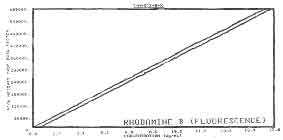
Figure 4. Calibration
Curve | 3.8 Safety
precautions (analytical)
3.8.1 Avoid skin contact and air
exposure to rhodamine B.
3.8.2 Avoid skin contact with
all solvents.
3.8.3 Wear safety glasses at all times.
4. Recommendation for
further study
This method should be fully validated.
5.
References
5.1 IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man; International
Agency for Research on Cancer: Lyon, 1978; Vol. 16, pp
221-231.
5.2 Registry of Toxic Effects of Chemical Substances 1985-86 Edition; U.S.
Department of Health and Human Services: Cincinnati, OH, 1987;
DHHS(NIOSH) Publication No. 87-114, p 313.
5.3 Merck Index, 10th ed.;
Windholz, Martha Ed.; Merck: Rathway, N.J., 1983; p 1180.
|

