1. General Discussion
1.1 Background
1.1.1 History of procedure This evaluation was undertaken
to determine the effectiveness of the OVS-2 tube as a sampling diuron
and to analyze samples. It follows the developed for carbaryl. (Ref.
5.1)
1.1.2 Toxic effects (This section is for information
only and should not be taken as the basis of OSHA policy). Herbicides
are weed killers with either general or selective applications in
agriculture. Herbicides interfere with plant chemistry and physiology.
They may inhibit plant respiration and photosynthesis, as well as
plant physiology by mimicking growth regulators or interfering with
their synthesis or action. Diuron is a herbicide whose chemical class
is phenyl urea. It has been shown to inhibit plant photosynthesis by
blocking light reaction II. Hence light is required to elicit its
phytotoxic effects. This herbicide demonstrates low acute toxicity
toward mammals. (Ref.
5.2) The following paragraph describing the toxicity is excerpted
from the book Documentation of the Threshold Limit
Values And Biological Exposure Indices (Ref.
5.3)
Hodge and co-workers have reported a low order of acute
and chronic toxicity for diuron. The oral LD5O for male
rats was given at 3400 mg/kg. No -effect dietary concentration
levels in two-year feeding studies are considered to be 250 ppm for
rats and 125 ppm for dogs. A dietary concentration of 125 ppm did
not adversely affect reproduction in a three-generation rat study.
There was no evidence of carcino genicity in these chronic studies
or in an 18-month study on mice at approximately 1400 ppm.
The following paragraph describing the harm and symptoms
of diuron were taken from the Handbook of Toxic and
Hazardous Chemicals and Carcinogens. (Ref.
5.4)
The concentrated material may cause irritation to the
eyes and mucous membranes, but a 50% water paste was not irritating
to the intact skin of guinea pigs. Due to these factors diuron has
been given a TLV-TWA of 10 mg/m3 by the ACGIH. (Ref.
5.3) OSHA adopted this same value as its PEL in
March 1989.
Editorial Note: These March 1989 PELs were vacated on
July 7, 1992 and ceased to be enforceable on March 23, 1993 (FR
58:35338-35351, 6/30/1993).
1.1.3 Potential workplace
exposure No estimate of worker exposure to diuron could be
found. Potential exposure involves those individuals in manufacturing,
formulation, and application of the herbicide. (Ref.
5.4) 1.1.4 Physical Properties (Ref.
5.2-5.7)
| Molecular weight: |
233.10 |
| Molecular Formula: |
C9H10Cl2N2O |
| CAS #: |
330-54-1 |
| IMIS #: |
2684 |
| Melting point: |
158 to 159°C |
| Vapor Pressure: |
0.0041 Pa (0.000031 mmHg)
at 30°C |
| Appearance: |
white crystalline
solid |
| Solubility: |
42 ppm in water at 25°C,
5.3% at 27°C, very low so'. hydrocarbon solvents |
| Synonyms: |
Cekiuron, Crisuron,
Dailon, Diater Di-on, Direx4L, Diurex, Diurol Dynex, Karmex, Rout,
Unidron, Urox Vonduron, dichlorfenidim (USSR) |
| Chemical names: |
3-(3,4-Dichlorophenyl)-1,1-dimethylurea;
N'-(3,4-dichlorophenyl)-N,N-dimethylurea |
| UV spectrum: |
 |
| Stability: |
Sunlight (ultraviolet
irradiation) degrades diuron. Decomposes on heating
(180-190°C) yielding dimethylamine and
3,4-dichlorophenylisocyanate. |
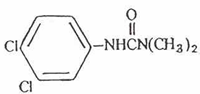
Structural Formula:  1.2 Limiting defining parameters The detection limit of
the analytical procedure is 1.81 ng per injection. This is the amount of
amount of analyte which will give a peak whose height is approximately
five times the baseline noise. 2.
Sampling Procedure
2.1 Apparatus
2.1.1 A personal sampling pump that
can be calibrated to within ±5% of the recommended flow rate with the
sampling device in line.
2.1.2 OVS-2 tubes, which
are specially made 13-mm tubes o.d. glass tubes that are tapered to
6-mm o.d. They are packed with a 140-mg backup section and a 270-mg
sampling section of cleaned XAD-2. The backup section is retained by
two foam plugs and the sampling section is between one foam plug and a
13-mm diameter glass fiber filter. The glass fiber filter is held next
to the sampling section by a polytetrafluoroethylene (PTFE) retainer.
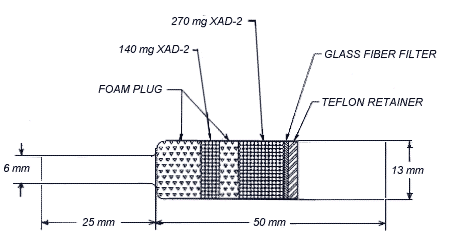
polytetrafluoroethylene (PTFE)
retainer | 2.2 Reagents
No sampling reagents are
required. 2.3 Sampling
Technique
2.3.1 Attach the small end of the
OVS-2 sampling tube to the sampling pump with flexible, plastic tubing
such that the large, front section of the sampling tube is exposed
directly to the atmosphere. Do not place any tubing in front of the
sampler.
2.3.2 Attach the sampler vertically (large end
down) in the worker's breathing zone in such a manner that it does not
impede work performance.
2.3.3 After sampling for the
appropriate time, remove the sampling device and seal the tube with
plastic end caps.
2.3.4 Wrap each sample end-to-end with
an OSHA seal (Form 21).
2.3.5 Submit at least one blank
with each set of samples. Handle the blank the same as the other
samples but do not draw air through it. 2.3.6 Submit any bulk
samples in a separate container. Do not ship them with the air
samples. 2.4 Extraction
efficiency Three OVS-2 tubes were each liquid spiked with 31 µL (1/20
PEL) of a 0.9627 mg/mL solution of diuron in acetonitrile. Three
additional OVS-2 tubes were each liquid spiked with 62 µL (1/10 PEL) of
the above diuron standard. These tubes were allowed to sit overnight on
a desk at ambient temperature and then extracted with 5.0 mL of
acetonitrile and analyzed as in Sections 3.4 and
3.5.
Table
2.4
OVS-2 Extraction Study
|
| Tubes # |
1/20 PEL |
1/10 PEL |
|
|
|
| OVS1 |
96.6% |
86.6% |
| OVS2 |
88.1% |
96.3% |
| OVS3 |
91.7% |
95.0% |
|
| Averages |
92.1% |
92.6% |
2.5
Retention efficiency Four OVS-2 tubes were each liquid spiked with 62 µL
of a 0.9627 mg/mL solution of diuron by spiking the glass fiber filter.
Sixty liters of humid air (approximately 70% relative humidity) were
drawn through each tube. Three of these tubes were then desorbed and
analyzed as in Sections 3.4 and 3.5. No diuron was recovered from the
backup section of these tubes. The fourth tube had 120 liters of humid
air drawn through it and had a recovery of 91.9%.
Table 2.5
Retention Efficiency Study |
| Tube # |
Recovery |
| RET1 |
95.6% |
| RET2 |
91.0% |
| RET3 |
95.5% |
|
| Average recovery is 94.0% |
2.6 Sample storage
Eighteen OVS-2 tubes were each
liquid spiked with 62 µL of a 0.9627 mg/mL solution of diuron by placing
it on the glass fiber filter. Sixty liters of humid air (approximately
70% relative humidity) were drawn through each tube. Half of the tubes
were stored in a drawer at ambient temperature, and the other half were
stored in a refrigerator (2°C). They were stored according to Table 2.6
and extracted and analyzed as in Section 3.4 and 3.5. No diuron was
recovered from the backup section of these tubes.
Table
2.6
Storage Study
|
| Days |
Ambient |
Averages |
Refrigerator |
Averages |
|
|
|
|
|
| 0 |
95.7% |
96.2% |
96.2% |
96.3% |
|
97.1% |
|
96.7% |
|
|
95.9% |
|
96.1% |
|
|
|
|
|
|
| 7 |
94.2% |
94.4% |
95.3% |
95.0% |
|
93.3% |
|
95.0% |
|
|
95.6% |
|
94.8% |
|
|
|
|
|
|
| 14 |
94.1% |
92.5% |
96.2% |
95.7% |
|
91.6% |
|
95.8% |
|
|
91.7% |
|
95.0% |
|
|
Average
recovery (ambient) 94.4%
Average recover (refrigerator) 95.7%
2.7 Recommended air volume and sampling
rate
2.7.1 The recommended air volume is 60
L.
2.7.2 The recommended flow rate is 1.0 L/min.
2.8 Interferences (sampling)
It
is not known if any compounds will interfere with the collection of
diuron. Suspected interferences should be reported to the laboratory
with submitted samples.
2.9 Safety precautions (sampling)
2.9.1 Attach the sampling equipment in
such a manner that it will not interfere with work performance or
employee safety.
2.9.2 Follow all safety practices that apply
to the work area being sampled. 3. Analytical Procedure
3.1 Apparatus
3.1.1 An HPLC equipped with a UV
detector and a manual or automatic injector. A Waters 600 pump, Waters
712 autosampler and Waters 490E UV detector were used in this
evaluation.
3.1.2 An HPLC column capable of separating diuron
from any interferences. A (8-cm x 6.2-mm i.d.) Golden Series Zorbax
ODS (3 micron) column was used in this evaluation.
3.1.3
An electronic integrator or other suitable means of measuring detector
response. A Hewlett-Packard 3357 Data System was used in this
evaluation.
3.1.4 Vials, 4-mL and 20-mL glass with capped or
PTFE-lined septa.
3.1.5 Volumetric flasks, pipets, and
syringes. 3.2 Reagents
3.2.1 Acetonitrile, HPLC grade.
3.2.2 Water, HPLC grade. A Millipore Milli-Q system was used
to prepare the water in this evaluation.
3.2.3 Diuron. A
99.25% pure standard from EPA was used in this
evaluation. 3.3 Standard
preparation
Prepare stock standard solutions by adding
acetonitrile to pre-weighed amounts of diuron. Prepare working range
standards by diluting stock solutions with acetonitrile. Store stock and
dilute standards in a freezer.
3.4 Sample preparation
3.4.1 Transfer the 13-mm glass fiber
filter and the 270-mg sampling section of the OVS-2 tube to a 20-mL
vial. Place the first foam plug and the 140-mg backup section in a
separate vial. A small glass funnel can be used to facilitate the
transfer of the adsorbent. Discard the rear foam plug. Do not discard
the glass sampling tube; it can be reused.
3.4.2 Add 5.0 mL of
acetonitrile to each vial.
3.4.3 Seal the vials and shake them
for half an hour on a mechanical shaker.
3.4.4 Transfer an
aliquot of sample to the 4-mL vial and seal with PTFE-lined
septa. 3.5 Analysis
3.5.1 Liquid chromatographic
conditions
| Column: |
8-cm x 6.2-mm i.d.
stainless steel Golden Series column packed with 3 micron Zorbax
ODS |
| Mobile Phase: |
55% acetonitrile / 45%
water |
| Flow Rate: |
1 mL/min |
| UV detector: |
254 nm |
| Retention time: |
5.44 min |
| Injection volume: |
15
µL |
3.5.2
Chromatogram
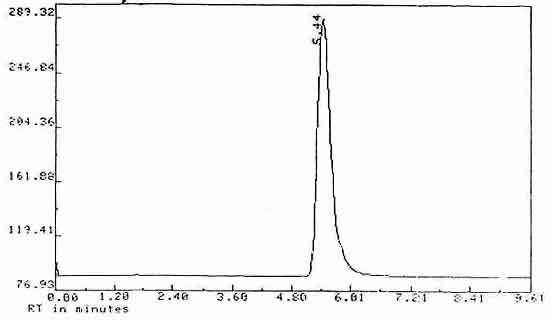
Figure 3. Chromatogram of Diuron
|
3.6 Interferences (analytical)
3.6.1 Any compound having a retention
time similar to that of the analyte is a potential interference.
Generally, chromatographic conditions can be altered to separate
interferences from the analyte.
3.6.2 Retention time on a
single column is not proof of chemical identity. Analysis by an
alternate HPLC column, detection at another wavelength (for comparison
of absorbance response ratios) and confirmation by mass spectrometry
are additional means of identification. 3.7 Calculations
3.7.1 Construct a calibration curve by
plotting detector response versus standard concentration.
3.7.2 Determine the concentration of diuron in each sample
from the calibration curve. If diuron is found on the backup section,
make blank corrections for each section separately before adding the
results together.
3.7.3 Determine the air concentration by the
following formula.
| mg/m3 = |
(µg/mL, blank corrrected) x
(desorption volume, mL)
(air volume, L) x (desorption efficiency, decimal)
|
3.8
Safety precautions (analytical)
3.8.1 Avoid exposure to all standards.
3.8.2 Avoid exposure to all solvents.
3.8.3 Wear
safety glasses at all times. 4. Recommendations for Further Study
4.1 A better desorption solvent than
acetonitrile might be found.
4.2 This method should be fully
validated.
4.3 This method has been partially evaluated at 60
liters or air at 1 liter per minute; however, since the PEL of diuron is
high and its solubility is low, it might be better to lower the sampling
rate to 200 cc/min to prevent the sampling tube from becoming clogged
with diuron.
5. References
5.1 Burright, D.; Method #63,
"Carbaryl"; OSHA Analytical Laboratory unpublished, 1987.
5.2
Cawse, J.N.; "Kirk-Othmer Encyclopedia of Chemical Technology", 3rd ed.;
John Wiley and Sons: New York, NY., 1980; Vol. 12, pp 297-322.
5.3 Documentation of the Threshold Limit Values and Biological
Exposure Indices", 5th ed.; American Conference of Governmental
Industrial Hygienists: Cincinnati, OH, 1986; p 228.
5.4 Sittig,
M.; "Handbook of Toxic and Hazardous Chemical and Carcinogens", 2nd ed.;
Noyes Publication: Park Ridge, NJ., 1985; p 394.
5.5 "Farm
Chemicals Handbook"; Meister Publishing Co.: Willoughby, OH, 1986; p
C88.
5.6 "Merck Index", 10th ed.; Windholz, M., Ed.; Merck and
Co.: Rahway, NJ, 1983; p 494.
5.7 Cawse, J.N.; "Kirk-Othmer
Encyclopedia of Chemical Technology", 3rd ed.; John Wiley and Sons: New
York, NY., 1980; vol. 21 pp 273-276.
|

