1. General Discussion
1.1 Background
1.1.1 History
Information on
Hexamethylene Diisocyanate Homopolymer or HDI Homo (Desmodur 3300
manufactured by Bayer) was not available. It was decided to follow the
OSHA Method 421
for Diisocyanates. HDI Homo is not available in pure
form.
1.1.2 Toxic effects (This section is for information only
and should not be taken as the basis of OSHA
policy.)
Recommended Manufacturer Guideline Level (MGL) is 0.5
mg/m³. This product is not listed by NTP, IRAC or regulated as a
carcinogen by OSHA. Desmodur 3300 aerosols at concentrations above MGL
can irritate (burning sensation). The mucus membranes in the
respiratory tract (nose, throat, lungs) causing runny nose, sore
throat, coughing, chest discomforts, shortness of breath and reduced
lung function. Exposures well above MGL may lead to bronchitis
,bronchial spasm and pulmonary edema ( fluid in lungs ). Chemical or
hypersensitive pneumonitis, with flu like symptoms (e.g. fever,
chills) has also been reported. Oral LD50 is >10000
mg/kg (rats), Dermal LD50 > 5000 mg/kg (rabbits)2.
1.1.3
Workplace exposure
No exposure data is available at present,
but this product is widely used in automobile industry in polyurethane
paint.
1.1.4 Physical properties and other descriptive
information2
| CAS number: |
28182-81-2 |
IMIS: |
H130 |
| molecular weight: |
approx 500 |
|
|
| boiling point: |
decomposes |
Wavelength: |
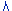 max - 254
nm max - 254
nm |
| appearance: |
yellowish liquid |
molecular formula: |
C24H36N6O6 |
| odor: |
odorless |
|
|
| synonyms: |
polymeric hexamethylene
diisocyanate; HDI Homo |
| solubility: |
soluble in methylene chloride,
decomposes in water and librates carbon
dioxide |
This method was evaluated according to the OSHA SLTC
"Evaluation Guidelines for Air Sampling Methods Utilizing Chromatographic
Analysis"3.
The Guidelines define analytical parameters, specify required laboratory
tests, statistical calculations and acceptance criteria. The analyte air
concentrations throughout this method are based on the recommended
sampling and analytical parameters. Air concentrations listed in ppm are
referenced to 25°C and 101.3 kPa (760 mmHg).
1.2 Detection Limit of the Overall
Procedure (DLOP) and Reliable Quantitation Limit (RQL):
The DLOP
is measured as mass per sample and expressed as equivalent air
concentrations, based on the recommended sampling parameters. Ten
samplers were spiked with equal descending increments of 1,6 -
Hexamethylene Diisocyanate Homopolymer such that the highest sampler
loading was 0.375 µg/sample. This is the amount spiked on a sampler that
would produce a peak approximately 10 times the response for a sample
blank. These spiked samplers and the sample blank were analyzed with the
recommended analytical parameters, and the data obtained used to
calculate the required parameters (Standard Error of Estimate and Slope)
for the calculation of the DLOP. Values of 4.65E04X-1.37E03 and 1570.14
were obtained for the slope and standard error of estimate respectively.
DLOP was calculated to be 0.1 µg/sample ( 0.0066 mg/m³).
Table
1.2
Detection Limit of the Overall Procedure
|
mass per
sample
(µg) |
area
counts
(µV-s) |
|
0
0.15
0.175
0.200
0.225
0.250
0.275
0.300
0.325
0.350
0.375 |
0
5470
7356
7593
8312
8743
9976
11265
12413
17296
18444 |
| |
Figure
1.2.1 Plot of data to determine the DLOP/RQL. (Y = 4.65E04X
- 1.37E03) |
| The RQL is considered the lower limit
for precise quantitative measurements. It is determined from the
regression line parameters obtained for the calculation of the
DLOP, providing 75% to 125% of the analyte is recovered. The RQL
is 0.34 µg per sample (0.023 mg/m³). Recovery at this
concentration is 84.5%. |
|
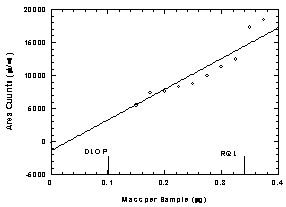
Figure
1.2.2 Chromatogram of the RQL for HDI
Homo. | 2.
Sampling Procedure
All safety practices that apply to the work area
being sampled should be followed. The sampling equipment should be
attached to the worker in such a manner that it will not interfere with
work performance or safety.
2.1 Apparatus
2.1.1 Samples are collected by use
of a personal sampling pump that can be calibrated to within ±5%
at the recommended flow rate with the sampling device in
line.
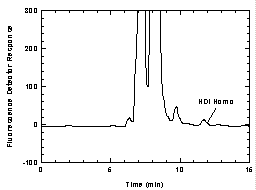
2.1.2 A three-piece styrene cassette containing a
glass fiber filter coated with 1.0 mg of 1-2PP and a backup pad.
(Figure 2.1.2)
2.1.3 Coated filters are prepared by
applying 0.5 ml of a solution of 0.2 mg/ml 1-2PP in methylene
chloride to each glass fiber filter. The wet filters are allowed
to air dry before placing them in a jar. Vacuum is applied to
the jar to remove residual methylene chloride.
|
|
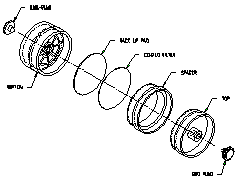
Figure
2.1.2 An illustration of the assembly of the filters in
the cassette. |
| 2.1.4 Coated filters should be
stored at refrigerated temperature as a
precaution. | 2.2 Reagents
None
required.
2.3 Technique
2.3.1 Immediately before sampling,
remove the top piece and the end plug from the cassette.
2.3.2
Attach the cassette to the sampling pump so that it is in an
approximately vertical position with the inlet facing down during
sampling. Position the sampling pump, cassette and tubing so it does
not impede work performance or safety.
2.3.3 Air being sampled
should not pass through any hose or tubing before entering the
cassette.
2.3.4 After sampling for the appropriate time, remove
the sample, and replace the top piece and the end plugs. Wrap each
sample end-to-end with a Form OSHA-21 seal.
2.3.5 Submit at
least one blank sample with each set of samples. Handle the blank
sampler in the same manner as the other samples except draw no air
through it.
2.3.6 Record sample volumes (in liters of air) for
each sample, along with any potential interferences.
2.3.7 Ship
any bulk samples separate from the air samples.
2.3.8 Submit
the samples to the laboratory for analysis as soon as possible after
sampling. If delay is unavoidable, store the samples in a
refrigerator 2.4 Extraction
efficiency
The extraction efficiency of 1,6-hexamethylene
diisocyanate homopolymer was determined by liquid-spiking glass fiber
filter coated with 1.0 mg of 1-2PP with the analyte over the range of
0.5 to 2 times the target concentration. These samples were stored
overnight at ambient temperature and then extracted with ACN/DMSO and
analyzed. The mean extraction efficiency over the studied range was
98.3% for HDI Homopolymer.
Table 2.4
Extraction
Efficiency of Hexamethylene Diisocyanate Homopolymer
|
| level |
|
sample
number |
|
|
× target
conc. |
µg per
sample |
1 |
2 |
3 |
4 |
mean |
|
0.1
0.2
0.5
1.0
2.0 |
0.75
1.50
3.75
7.50
15.0 |
96.7
105.7
99.2
99.0
99.5 |
103.3
102.1
95.3
98.6
97.6 |
101.2
102.2
96.6
100.9
97.5 |
96.5
92.5
96.9
91.3
94.2 |
99.4
100.6
97.0
97.5
97.2 |
| average |
|
|
|
|
|
98.3 |
|
2.5 Retention
efficiency
Five glass fiber filter coated with 1.0 mg of 1-2PP
were spiked with 15 µg 1,6-hexamethylene diisocyanate homopolymer,
allowed to equilibrate for 6 h, and then had 15 L humid air (absolute
humidity of 15.9 mg/L of water, about 80% relative humidity at 22.2°C)
pulled through them. The samples were extracted with ACN / DMSO and
analyzed. The mean retention efficiency is 96.2 %.
Table 2.5
Retention
Efficiency of Hexamethylene Diisocyanate Homopolymer
|
| |
|
|
sample
number |
|
|
| section |
1 |
2 |
3 |
4 |
5 |
mean |
|
| |
94.2 |
99.4 |
95.4 |
96.2 |
95.8 |
96.2 |
|
2.6 Sample storage
Nine glass
fiber filter coated with 1.0 mg of 1-2PP were each spiked with 7.5
µg of 1,6-hexamethylene diisocyanate homopolymer. They had 15 L of
air with an absolute humidity of 15.7 milligrams of water per
liter of air (about 80% relative humidity at 22.2°C) drawn through
them. They were sealed and stored at room temperature. Three
samples were analyzed immediately. Three more were analyzed after
7 days of storage and the remaining three after 18 days of
storage. The amounts recovered, which are corrected for extraction
efficiency, indicate good storage stability for the time period
studied. |
Table
2.6
Storage Test for Hexamethylene
diisocynate
homopolymer
|
| |
sample |
| time (days) |
1 |
2 |
3 |
0
7
18 |
97.9
98.0
93.7 |
97.4
101.9
96.0 |
99.9
98.8
98.1 |
| |
2.7 Recommended air volume and sampling rate
Based
on the data collected in this evaluation, 15-L air samples should be
collected at a sampling rate of 1.0 L/min. 3. Analytical Procedure
Adhere to the rules set down
in your Chemical Hygiene Plan4.
Avoid skin contact and inhalation of all chemicals.
3.1 Apparatus
3.1.1 A liquid chromatograph equipped
with an ultraviolet and fluorescence detector. A Waters 600E
controller,490E ultraviolet detector, spectraflow 980 fluorescence and
717 autosampler was used in this evaluation.
3.1.2 An LC column
capable of separating the analyte from any interferences. The column
used in this study was a Econosphere CN, 5-µm particle, 15-cm long
with 4.6-mm i.d. An alternate column is Altech CN 5-µm particle, 25-cm
long with 4.6-mm i.d.
3.1.3 An electronic integrator or some
suitable method of measuring peak areas. A Waters
Millennium32 data system was used in this
evaluation.
3.1.4 Four milliliter glass vials with PTFE-lined
caps.
3.1.5 Volumetric flasks - 10-mL and other convenient
sizes for preparing standards.
3.1.6 Pipets for dispensing the
extracting solve 3.2
Reagents
3.2.1 Methylene chloride, Fisher
Scientific Optima lot # 964274.
3.2.2 Acetonitrile, Fisher
Scientific HPLC grade lot # 964274.
3.2.3 Dimethyl sulfoxide,
JT Baxter lot # BB 470.
3.2.4 Deionized water. A Millipore
Milli-Q water purification system.
3.2.5 Phosphoric acid. JT
Baker analyzed phosphoric acid 85.9%, (lot D25821).
3.2.6
1-(2-Pyridyl)piperzine , Aldrich, Milwaukee, WI.
3.2.7 Ammonium
acetate, HPLC grade Fisher Scientific lot # 89784.
3.2.8
Glacial acetic acid, JT Baker analyzed lot# 18973.
3.2.9
Desmodur N 3300 , Bayer lot# Bn 43671. 3.3 Standard preparation
The concentration of
Desmodur N 3300 weighed out into a 10 mL flask was 15 mg, and methylene
chloride was added to the mark. This stock standard was used to make
working range standards by spiking on 1-2PP coated GFF, and extracting
with 3 mL of 90/10 (v/v) ACN/DMSO.
3.4 Sample preparation
3.4.1 The polystyrene cassette is
opened and the glass fiber filter is placed into a 4-mL vial so that
the filter is flat against the inside surface of the vial, not folded
or crumpled.
3.4.2 Three milliliters of the extracting
solution, 90/10 (v/v) ACN/DMSO, are added.
3.4.3 A cap equipped
with a Teflon liner is installed.
3.4.4 The vial is shaken to
remove large air bubbles from between the filter and the glass. Let
the vial set for 1 h. 3.5
Analysis
3.5.1 Reverse phase HPLC
conditions
| column: |
Econosphere CN , 5-µm particle,
15-cm long with 4.6-mm i.d. |
| mobile phase: |
0.01 M ammonium acetate in 60/ 40
ACN/water (v/v) adjusted to pH 6.0 with acetic acid |
| flow rate: |
0.7 mL/min |
| UV detector: |
254 nm |
| fluorescence detector: |
240 nm excitation (370
nm emission) |
| injection size: |
20 µL |
Reverse phase HPLC conditions (alternate condition for
general Diisocyanates)
| column: |
Altech CN , 5-µm particle, 25-cm
long with 4.6-mm i.d. |
| mobile phase: |
0.01 M ammonium acetate in 42/ 58
ACN/water (v/v) adjusted to pH 6.0 with acetic acid |
| flow rate: |
1.0 mL/min |
| UV detector: |
254 nm |
| fluorescence detector: |
240 nm excitation (370
nm emission) |
| injection size: |
20 µL |
| chromatogram: |
Figure 3.5.1 |
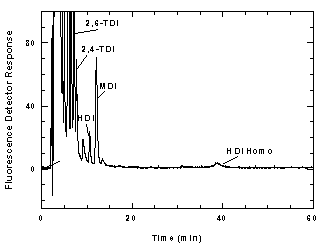
Figure
3.5.1 A chromatogram of an analytical standard at the
target concentration. |
3.5.2 An external standard procedure is used to prepare
a calibration curve using at least 2 stock solutions from which
dilutions are made. The calibration curve is prepared daily. The
samples are bracketed with analytical standards.
3.6 Interferences (analytical)
3.6.1 Any compound having the same
retention time as the analyte is a possible interference. Generally,
chromatographic conditions can be altered to separate an
interference.
3.6.2 Retention time on a single column is not
proof of chemical identity. Analysis by an alternate column system,
ratioing of wavelength response, and mass spectrometry are additional
means of identity. 3.7
Calculation
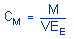 |
where |
CM is concentration by weight
(mg/m³)
M is micrograms per
sample
V is liters of air
sampled
EE is extraction
efficiency, in decimal form | 4. Recommendations for Further Study
Collection and
reproducibility study needs to be performed.
1. Burright, D OSHA Method 42, DIISOCYANATES, http://www.osha-slc.gov/index.html
(02/02/2003 accessed).
2. Material safety data sheet ,Desmodur N
3300 , Bayer approval date : 04/06/200.
3. Burright, D.; Chan, Y.;
Eide, M.; Elskamp, C.; Hendricks, W.; Rose, M. C. Evaluation Guidelines for Air Sampling Methods Utilizing
Chromatographic Analysis; OSHA Salt Lake Technical Center, U.S.
Department of Labor: Salt Lake City, UT, 1999.
4. Occupational
Exposure to Hazardous Chemicals in Laboratories. Code
of Federal Regulations, Part 1910.1450, Title 29,
1998.
|

