| Safety and Health
Topics > Sampling
and Analytical Methods > Index |
 Printing Instructions Printing Instructions |
1,6-Hexanediol
Diacrylate
|
|
| Method no.: |
PV2133 |
| |
|
| Control no.: |
T-PV2133-01-0403-M |
| |
|
| Target
Concentration: |
1 mg/m3
(AIHA Workplace Environmental Exposure Level (WEEL)) |
| |
|
| Procedure: |
Samples are collected
by drawing a known volume of air through glass sampling tubes
containing Chromosorb 106. Samples are extracted with 99:1
carbon disulfide:N,N-dimethylformamide and analyzed by GC
using a flame ionization detector. |
| |
|
| Recommended sampling
time and sampling rate: |
240 min at 0.2 L/min
(48 L) |
| |
|
| Reliable quantitation
limit: |
43 µg/m3 |
| |
|
| Status of method: |
Partially evaluated
method. This method has been subjected to established
evaluation procedures of the Methods Development Team and is
presented for information and trial use. |
| |
|
| March 2004 |
Mary
Eide |
| |
|
Methods Development Team
Industrial Hygiene
Chemistry Division
OSHA Salt Lake Technical Center
Sandy
UT 84070-6406
|
1.
General Discussion
1.1 Background
1.1.1 History
Air samples collected
using Chromosorb 106 tubes were received at OSHA SLTC with
requested analysis for 1,6-hexanediol diacrylate. This
partially-validated work was performed because SLTC had no
sampling and analytical method for 1,6-hexanediol
diacrylate.
The result of a preliminary extraction
efficiency study for 1,6-hexanediol diacrylate extracted
from dry Chromosorb 106 with carbon disulfide was 99%
recovery. The test was repeated with wet Chromosorb 106
and the recovery was initially the same, but then
decreased to 85.2% when the samples were allowed to stand.
The source of dry Chromosorb 106 was sampling tubes as
received from SKC Inc. The source of wet Chromosorb 106
was dry Chromosorb 106 sampling tubes which had clean,
humid air drawn through them. The extraction solvent was
changed from pure carbon disulfide to 99:1 carbon
disulfide:N,N-dimethylformamide
and the wet Chromosorb 106 extraction efficiency test was
repeated. The results of this test showed no decrease in
recovery, therefore, the 99:1 carbon disulfide:N,N-dimethylformamide extraction
solvent was selected for use in this work. The extraction
efficiency was 98.9% using the 99:1 carbon disulfide:N,N-dimethylformamide extraction
solvent.
1,6-Hexanediol diacrylate was found to be
well retained on Chromosorb 106, with a retention
efficiency recovery of 98.7% and the storage stability
recovery of 97.3% on day 14 of ambient storage.
1.1.2 Toxic effects (This section is for
information only and should not be taken as the basis of
OSHA policy.)1,2
1,6-Hexanediol
diacrylate is a contact irritant severely affecting the
skin, eyes, and respiratory system. It is moderately toxic
by skin contact and may cause sensitization.
1.1.3
Workplace exposure3
1,6-Hexanediol diacrylate is used as a
cross-linking agent in UV curing, inks and coatings.
1.1.4 Physical properties and other descriptive
information4,5
| CAS number: |
13048-33-4 |
| synonyms: |
acrylic acid hexamethylene ester;
hexaneglycol diacrylate; Kayard HDDA; Photomer 4017;
propenoic acid, 1,6-hexanediol ester; 2-propenoic
acid, 1,6-hexanediyl ester; Setalux UV 2243; Viscoat
230 |
| IMIS:6 |
H128 |
| RTECS: |
AT1430000 |
| molecular weight: |
226.3 |
| density (g/mL): |
1.01 |
| melting point: |
5
°C |
| boiling point: |
107 °C |
| appearance: |
clear liquid |
| flash point: |
132 °C (270 °F) (Cleveland open cup) |
| odor: |
mild ester-like |
| molecular formula: |
C12H10O4 |
| solubility: |
acetone, alcohol, benzene, and carbon
tetrachloride |
| autoignition temperature: |
235 °C (455
°F) |
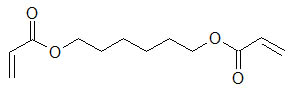
structural formula:
This method was evaluated according to the
OSHA SLTC "Evaluation Guidelines for Air Sampling Methods
Utilizing Chromatographic Analysis"7.The
Guidelines define analytical parameters, specify required
laboratory tests, statistical calculations and acceptance
criteria. The analyte air concentrations throughout this
method are based on the recommended sampling and analytical
parameters.
1.2 Detection limit of the overall procedure
(DLOP) and reliable quantitation limit (RQL)
The
DLOP is measured as mass per sample and expressed as
equivalent air concentration, based on the recommended
sampling parameters. Ten samplers were spiked with equal
descending increments of analyte, such that the highest
sampler loading was 8.08 µg of 1,6-hexanediol diacrylate.
This is the amount spiked on a sampler that would produce a
peak at least 10 times the response for a sample blank.
These spiked samplers were analyzed with the recommended
analytical parameters, and the data obtained used to
calculate the required parameters (standard error of
estimate and slope) for the calculation of the DLOP. The
slope was 1007 and the SEE was 234. The RQL is considered
the lower limit for precise quantitative measurements. It is
determined from the regression line parameters obtained for
the calculation of the DLOP, providing 75% to 125% of the
analyte is recovered. The DLOP and RQL were 0.70 µg and 2.32
µg, respectively. The recovery at the RQL was 98.2%.
Table 1.2
Detection Limit of the Overall
Procedure for 1,6-Hexanediol Diacrylate
|
mass per sample
(µg) |
area counts
(µV-s) |
|
| 0.00 |
0 |
| 0.81 |
721 |
| 1.62 |
1230 |
| 2.42 |
1863 |
| 3.23 |
2592 |
| 4.04 |
3586 |
| 4.85 |
4360 |
| 5.66 |
5341 |
| 6.46 |
6143 |
| 7.27 |
7105 |
| 8.08 |
8089 |
|
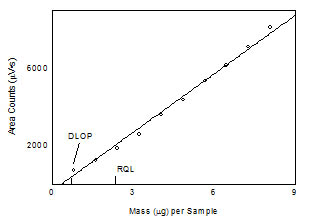
Figure 1.2.1 Plot of data to determine
the DLOP/RQL for 1,6-hexanediol diacrylate. (y = 1007x
- 337) |
Below is a chromatogram of 1,6-Hexanediol near
the RQL. The recovery was 89.2% at this level.
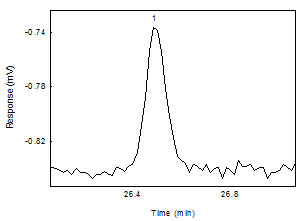
Figure 1.2.2 Chromatogram of
1,6-hexanediol diacrylate peak near the RQL. (Key: (1)
1, 6-hexanediol diacrylate)
| 2.
Sampling Procedure
All safety practices that apply to
the work area being sampled should be followed. The sampling
equipment should be attached to the worker in such a manner
that it will not interfere with work performance or safety.
2.1 Apparatus
2.1.1 Samples are collected using a personal
sampling pump calibrated, with the sampling device
attached, to within ±5% of the recommended flow rate.
2.1.2 Samples are collected with 7-cm × 4-mm i.d.
× 7-mm o.d. glass sampling tubes packed with two sections
(100/50 mg) of Chromosorb 106. The sections are held in
place with foam plugs and with a glass wool plug at the
front. For this evaluation, commercially prepared sampling
tubes were purchased from SKC, Inc. (Catalog no. 226-110,
lot 2573). 2.2 Reagents
None required.
2.3 Technique
2.3.1 Immediately before sampling, break off
the ends of the flame-sealed tube to provide an opening
approximately half the internal diameter of the tube. Wear
eye protection when breaking the tube. Use tube holders to
minimize the hazard of broken glass. All tubes should be
from the same lot.
2.3.2 The smaller section of
the adsorbent tube is used as a back-up and is positioned
nearest the sampling pump. Attach the tube holder to the
sampling pump so that the adsorbent tube is in an
approximately vertical position with the inlet facing down
during sampling. Position the sampling pump, tube holder
and tubing so they do not impede work performance or
safety.
2.3.3 Draw the air to be sampled directly
into the inlet of the tube holder. The air being sampled
is not to be passed through any hose or tubing before
entering the sampling tube.
2.3.4 After sampling
for the appropriate time, remove the adsorbent tube and
seal it with plastic end caps. Seal each sample end-to-end
with an OSHA-21 form as soon as possible.
2.3.5
Submit at least one blank sample with each set of samples.
Handle the blank sample in the same manner as the other
samples except draw no air through it.
2.3.6
Record sample air volumes (liters), sampling time
(minutes), and sampling rate (L/min) for each sample,
along with any potential interference on the OSHA-91A
form.
2.3.7 Submit the samples to the laboratory
for analysis as soon as possible after sampling. If delay
is unavoidable, store the samples at refrigerator
temperature. Ship any bulk samples separate from the air
samples.
2.4 Extraction efficiency
The extraction efficiency was determined by spiking
the front sections of Chromosorb 106 tubes with 1,
6-hexanediol diacrylate at 0.1 to 2 times the target
concentration, based on a 48-L air volume, for a loading of
4.85 to 97.0 µg/sample. These samples were stored overnight
at ambient temperature and then extracted with 1 mL of
extracting solvent on a shaker for 30 minutes, and analyzed
by GC-FID. The mean extraction efficiency over the studied
range was 98.9%. The wet extraction efficiency was
determined at 1 times the target concentration by liquid
spiking the analyte onto Chromsorb 106 tubes which had 48-L
humid air (absolute humidity of 15.9 mg/L of water, about
80% relative humidity at 22.2 °C) drawn through them
immediately before spiking. The mean recovery for the wet
samples was 98.7%.
Table 2.4
Extraction Efficiency
(%) of 1,6-Hexanediol Diacrylate |
|
level
|
sample number
|
mean
|
|
x target
concn |
µg per
Sample |
1 |
2 |
3 |
4 |
5 |
6 |
|
|
| 0.1 |
4.85 |
98.7 |
99.3 |
97.9 |
97.6 |
99.0 |
99.1 |
98.6 |
| 0.5 |
24.3 |
99.8 |
99.9 |
99.4 |
98.7 |
99.9 |
99.7 |
99.6 |
| 1.0 |
48.5 |
98.9 |
99.0 |
98.3 |
98.0 |
98.5 |
98.2 |
98.5 |
| 1.5 |
72.7 |
99.0 |
97.9 |
98.7 |
98.3 |
98.7 |
98.9 |
98.6 |
| 2.0 |
97.0 |
98.7 |
99.2 |
99.0 |
98.9 |
99.1 |
99.2 |
99.0 |
| |
| 1.0 (wet) |
48.5 |
97.9 |
98.7 |
98.9 |
98.8 |
99.3 |
98.3 |
98.7 |
|
2.5
Retention efficiency
Six Chromosorb 106 tubes were
spiked with 97.0 µg (2.02 mg/m3) of
1,6-hexanediol diacrylate in the front sections, and allowed
to equilibrate for 4 h. The tubes had 48-L humid air
(absolute humidity of 15.9 mg/L of water, about 80% relative
humidity at 22.2 °C) pulled through them at 0.2 L/min. The
samples were extracted and analyzed. The mean recovery was
98.7%. There was no analyte found on the back-up section of
any of the tubes.
Table 2.5
Retention Efficiency (%) of
1,6-Hexanediol
Diacrylate |
|
section
|
sample number
|
mean
|
| |
1 |
2 |
3 |
4 |
5 |
6 |
|
|
| front of spiked tube |
99.0 |
98.7 |
99.2 |
98.8 |
97.9 |
98.3 |
98.7 |
| rear of spiked tube |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
| total |
99.0 |
98.7 |
99.2 |
98.8 |
97.9 |
98.3 |
98.7 |
|
2.6 Sample
storage
Fifteen Chromosorb 106 tubes were each
spiked with 48.5 µg (1.01 mg/m3) of
1,6-hexanediol diacrylate. They were allowed to equilibrate
for 6h, then 48 L of air, with an absolute humidity of 15.7
milligrams of water per liter of air (about 80% relative
humidity at 23 °C), drawn through them. Three samples were
analyzed immediately, and the rest were sealed. Six samples
were stored at room temperature (23 °C), while the other six
samples were stored at refrigerated temperature (4 °C).
Three samples from each set of storage samples were analyzed
after 7 days of storage and the remaining three from each
set after 14 days. The amounts recovered indicate good
storage stability for the time period studied.
Table 2.6
Storage Test for
1,6-Hexanediol
Diacrylate |
|
| time
(days) |
ambient storage recovery (%) |
refrigerated storage recovery (%) |
|
| 0 |
98.5 |
99.0 |
99.4 |
|
|
|
| 4 |
98.1 |
98.6 |
97.8 |
98.8 |
99.1 |
98.2 |
| 14 |
96.9 |
98.0 |
97.0 |
98.2 |
98.9 |
97.8 |
|
2.7
Recommended air volume and sampling rate
Based on
the data collected in this evaluation, 48-L air samples
should be collected at a sampling rate of 0.2 L/min for 240
minutes.
2.8 Interferences (sampling)
2.8.1 There are no known compounds which will
severely interfere with the collection of 1,6-hexanediol
diacrylate.
2.8.2 Suspected interferences should
be reported to the laboratory with submitted samples.
3.
Analytical Procedure
Adhere to the rules set down in
your Chemical Hygiene Plan. Avoid skin contact and inhalation
of all chemicals and review all appropriate MSDSs.
3.1 Apparatus
3.1.1 A gas chromatograph equipped with an FID
detector. For this evaluation, an Agilent 6890 GC was
used.
3.1.2 A GC column capable of separating
1,6-hexanediol diacrylate from the extraction solvent,
internal standard, and any potential interferences. A 60-m
× 0.32-mm i.d. capillary column coated with DB-1 with a
1.0-µm df (J&W Scientific, Folsom, CA) was used in
this evaluation.
3.1.3 An electronic integrator or
some other suitable means of measuring peak areas. A
Waters Millennium32 Data System and an Agilent
3396 integrator were used in this evaluation.
3.1.4 Glass vials with
poly(tetrafluoroethylene)-lined caps. For this evaluation
2-mL vials were used.
3.1.5 A dispenser capable of
delivering 1.0 mL of extraction solvent to prepare
standards and samples. If a dispenser is not available, a
1.0-mL volumetric pipet may be used.
3.1.6
Volumetric flasks – 10-mL and other convenient sizes for
preparing standards.
3.1.7 Calibrated 10-µL
syringe for preparing standards.
3.1.8 A shaker or
rotator to agitate samples during extraction. An Eberbach
mechanical shaker was used in this evaluation.
3.2 Reagents
3.2.1 1,6-Hexanediol diacrylate, technical
grade. Aldrich 80% (lot 16304HA) was used in this
evaluation.
3.2.2 Carbon disulfide, Reagent grade.
EM Science Omni-Solv® 99.99% (lot 43279343), was used in
this evaluation.
3.2.3 N,N-Dimethylformamide, anhydrous.
Aldrich 99.8% (lot 04643LA) was used in this evaluation.
3.2.4 p-Cymene, reagent
grade. Aldrich 99% (lot 11703TR) was used in this
evaluation.
3.2.5 The extraction solvent was a
solution of 99:1 carbon disulfide:DMF with 0.25 µL/mL of
p-cymene as internal standard.
3.3 Standard preparation
3.3.1 Prepare at least two stock analytical
standards by injecting microliter quantities of
1,6-hexanediol diacrylate from a microliter syringe into
volumetric flasks containing the extraction solvent.
Working analytical standards are prepared by serial
dilutions of the stock standard with the extraction
solvent. A stock standard of 6 µL/10 mL (0.6 µL/mL) is
equivalent to 485 µg/mL, based on the density and the
purity of 80%. A 1:10 dilution of this stock standard is
48.5 µg/mL, which is equivalent to 1.01 mg/m3
based on a 48-L air volume.
3.3.2 Bracket sample
concentrations with standard concentrations. If sample
concentrations are higher than the concentration range of
prepared standards, either analyze higher standards, or
dilute the sample. Diluted samples should be prepared with
the extracting solvent to obtain a concentration within
the existing standard range. The range of standards used
in this study was from 1 to 121 µg/mL. A check standard
from a second source should be prepared to check the
calibration. 3.4 Sample preparation
3.4.1 Remove the plastic end caps from the
sample tubes and carefully transfer each adsorbent section
to separate 2-mL vials. Discard the glass tube, urethane
foam plug and glass wool plug.
3.4.2 Add 1.0 mL of
extraction solvent to each vial using the same dispenser
as used for preparation of standards.
3.4.3
Immediately seal the vials with
poly(tetrafluoroethylene)-lined caps.
3.4.4
Agitate the vials on a shaker or rotator for 30 minutes.
3.5 Analysis
3.5.1 Gas chromatographic conditions
GC conditions
|
| zone temperatures: |
initial 50°C, hold 1 min, ramp at 10°C/min
to 170°C, hold 17 min |
| injector: |
250 °C |
| detector: |
250 °C |
| run time: |
30 min |
| column gas flow: |
3.2 mL/min (hydrogen) |
| injection size: |
1.0 µL (10:1 split) |
| column: |
60-m × 0.32-mm i.d. capillary DB-1 (df = 1
µm) |
| retention times: |
4.0 min (CS2); 7.0 min (DMF);
11.8 min (p-cymene); 26.5
min (1,6-hexanediol diacrylate
|
FID conditions
|
| hydrogen flow: |
30
mL/min |
| air flow: |
400 mL/min |
| nitrogen makeup flow: |
25
mL/min |
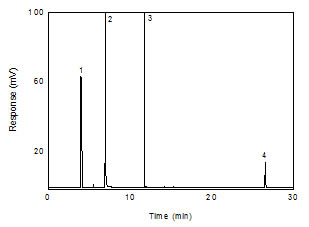
Figure 3.5.1 A chromatogram of 48.4 µg/mL
1,6-hexanediol diacrylate in 99:1 CS2:DMF
with 0.25 µl/mL p-cymene
internal standard. (Key: (1) CS2; (2)
DMF; (3) p-cymene; (4)
1,6-hexanediol
diacrylate) |
3.5.2 Peak areas are measured by an
integrator or other suitable means.
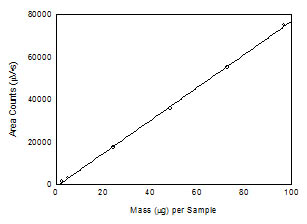
Figure 3.5.2 Calibration curve of 1,6-hexanediol
diacrylate. (y = 779x -
1057) |
3.5.3 An internal standard (ISTD)
calibration method is used. A calibration curve can be
constructed by plotting ISTD-corrected response of
standard injections versus micrograms of analyte per
sample. Bracket the samples with freshly prepared
analytical standards over the range of
concentrations. 3.6
Interferences (analytical)
3.6.1 Any compound that produces a GC response
and has a similar retention time as the analyte is a
potential interference. If any potential interferences
were reported, they should be considered before samples
are extracted. Generally, chromatographic conditions can
be altered to separate interference from the analyte.
3.6.2 When necessary, the identity or purity of an
analyte peak may be confirmed by GC-mass spectrometry or
by another analytical procedure. The mass spectrum in
Figure 3.6.2 was from the NIST spectral library.
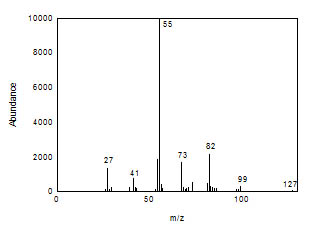
Figure 3.6.2 Mass spectrum of 1,6-hexanediol
diacrylate. | 3.7 Calculations
The amount of analyte
per sampler is obtained from the appropriate calibration
curve in terms of micrograms per sample, uncorrected for
extraction efficiency. This total amount is then corrected
by subtracting the total amount (if any) found on the blank.
The air concentration is calculated using the following
formula.
| where: |
CM is concentration by
weight (mg/m3) |
| M is micrograms per sample |
| V is liters of air sampled |
| EE is extraction
efficiency, in decimal
form |
4. Recommendations for further
study
Collection, reproducibility, and other detection
limit studies need to be performed to make this a fully
validated method.
References
1. Lewis, R., Sax's Dangerous Properties of Industrial
Materials, Tenth ed., Vol. 3, John Wiley & Sons
Inc., New York, 2000, p 1951.
2. Material Safety Data
Sheet: Hexanediol Diacrylate, Chemwatch, Victoria, Australia,
(accessed 12/17/03).
3. Lewis, R., Sax's Dangerous Properties of Industrial
Materials, Tenth ed., Vol. 3, John Wiley & Sons
Inc., New York, 2000, p 1951.
4. Lewis, R., Sax's Dangerous Properties of Industrial
Materials, Tenth ed., Vol. 3, John Wiley & Sons
Inc., New York, 2000, p 1951.
5. Material Safety Data
Sheet: 1,6-Hexanediol Diacrylate, Aldrich Chemical Co.,
Milwaukee, WI, (accessed 10/3/03).
6. OSHA Chemical
sampling Information, http://www.osha-slc.gov/
(accessed 12/17/03).
7. Burright, D.; Chan, Y.; Eide,
M.; Elskamp, C.; Hendricks, W.; Rose, M. C. Evaluation Guidelines for Air Sampling Methods
Utilizing Chromatographic Analysis; OSHA Salt Lake
Technical Center, U.S. Department of Labor: Salt Lake City,
UT, 1999.
|
| |
| |
| | |

